Fabrication of Silver Nanoparticles Dispersed in Palm Oil Using Laser Ablation
Abstract
:1. Introduction
2. Experimental
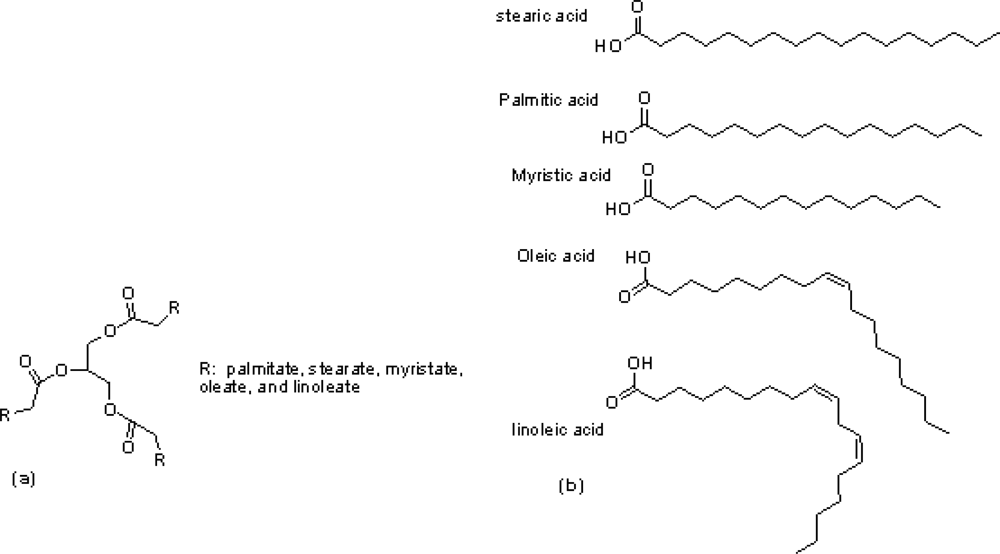
2.1. Materials
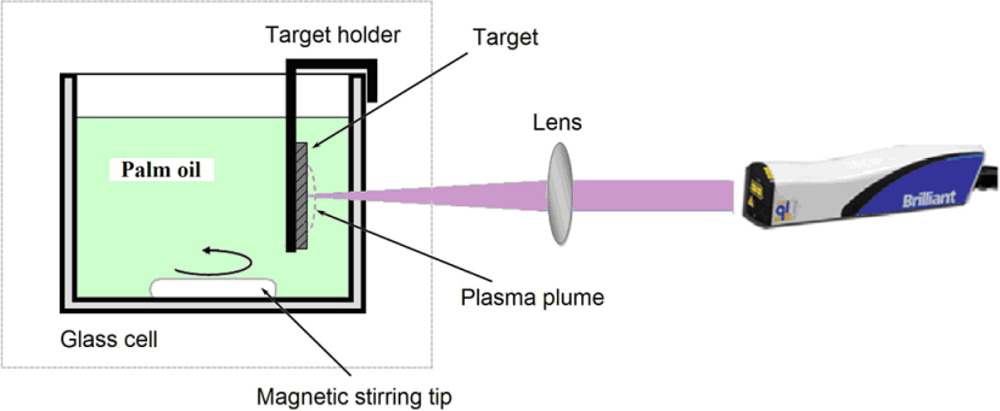
2.2. Synthesis
2.3. Characterization
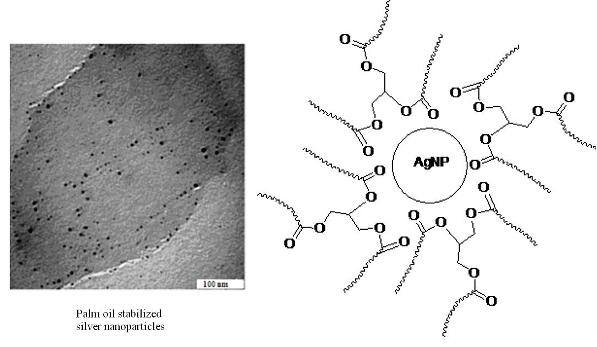
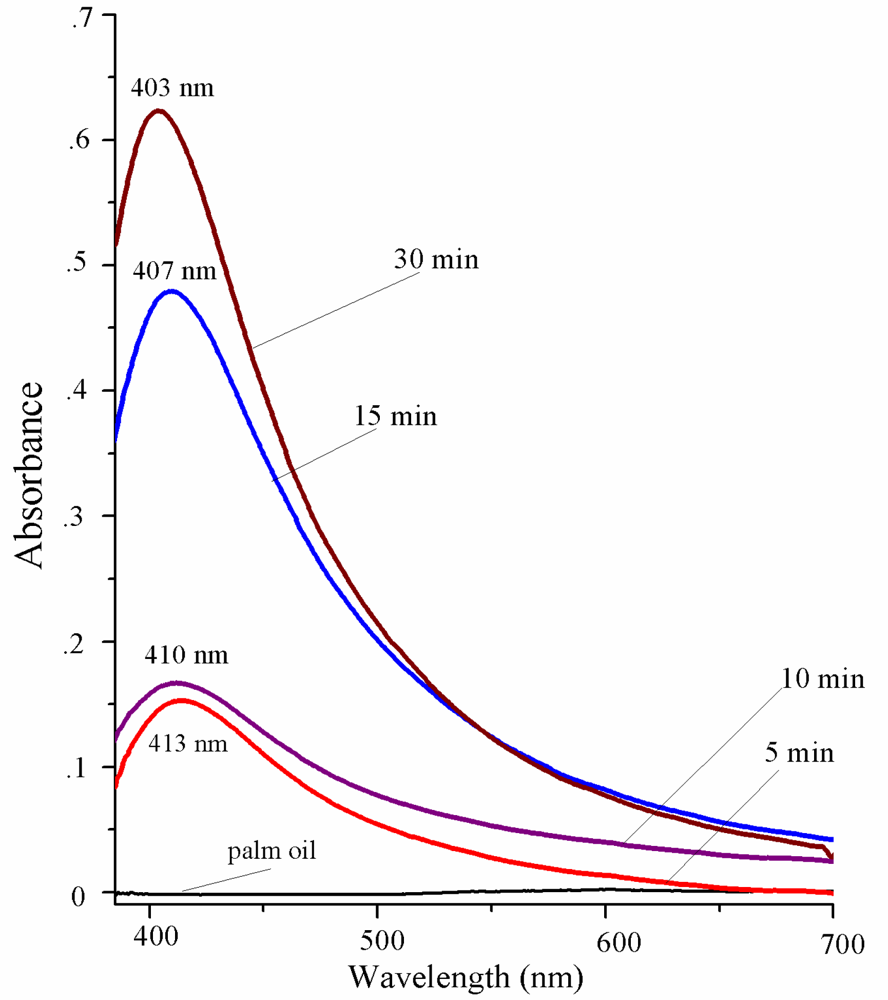
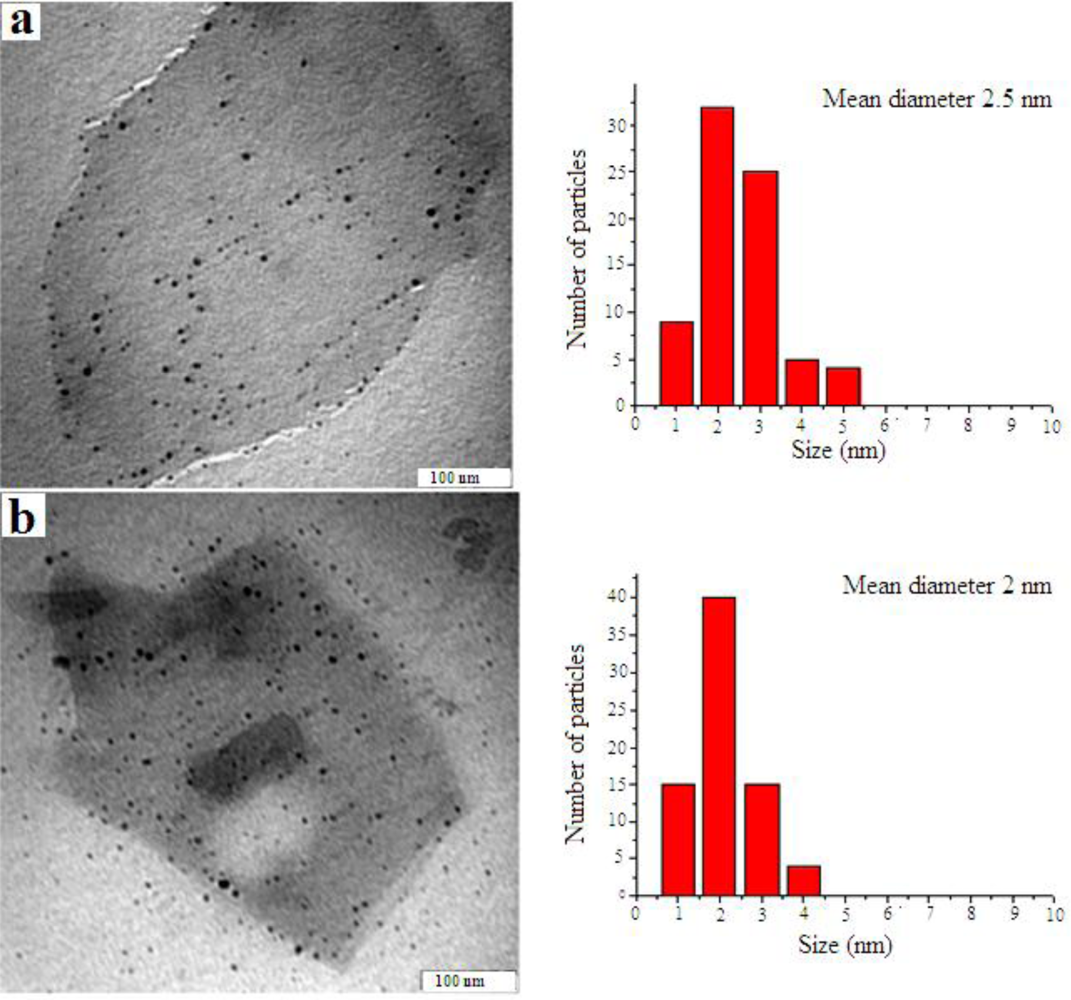
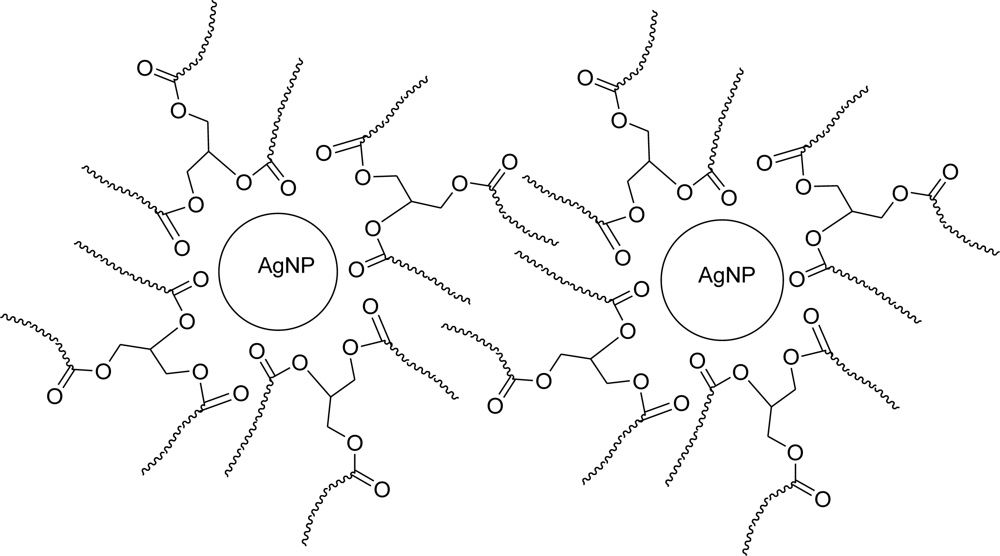
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Xiong, Y; Chen, J; Wiley, B; Xia, Y; Yin, Y; Li, Z. Size-Dependence of Surface Plasmon Resonance and Oxidation for Pd Nanocubes Synthesized via a Seed Etching Process. Nano Lett 2005, 5, 1237–1241. [Google Scholar]
- Renteria, VM; Garcia-Macedo, J. Modeling of optical absorption of silver prolate nanoparticles embedded in sol-gel glasses. Chem. Phys 2005, 91, 88–93. [Google Scholar]
- Diao, JJ; Sun, J; Hutchison, JB. Self assembled nanoparticle wires by discontinuous vertical colloidal deposition. Appl. Phys. Lett 2005, 87, 103113–103116. [Google Scholar]
- Tarasenko, NV; Butsen, AV; Nevar, EA. Laser-induced modification of metal nanoparticles formed by laser ablation technique in liquids. Appl. Surf. Sci 2005, 247, 418–422. [Google Scholar]
- Dowling, DP; Betts, AJ; Pope, C; McConnell, ML; Eloy, R; Arnaud, MN. Anti-bacterial silver coatings exhibiting enhanced activity through the addition of platinum. Surf. Coat. Technol 2003, 637, 163–164. [Google Scholar]
- Song, CD; Wang, Y; Lin, Z; Hu, G; Gu, X; Fu, X. Formation of silver nanoshells on latex spheres. Nanotechnology 2004, 15, 962–965. [Google Scholar]
- Kokkoris, M; Trapalis, CC; Kossionides, S; Vlastou, R; Nsouli, B; Grötzschel, R; Spartalis, SG; Kordas, T. RBS and HIRBS studies of nanostructured AgSiO2 sol-gel thin coatings. Nucl. Instrum. Methods Phys. Res. B 2005, 188, 67–72. [Google Scholar]
- Roucoux, A; Schulz, J; Patin, H. Reduced transition metal colloids: a novel family of reusable catalysts. Chem. Rev 2002, 102, 3757–3778. [Google Scholar]
- Stoeva, SI; Smetana, AB; Sorensen, CM; Klabunde, KJ. Gram-scale synthesis of aqueous gold colloids stabilized by various ligands. J. Colloid Interface Sci 2007, 309, 94–98. [Google Scholar]
- Wu, N; Fu, L; Aslam, M; Wong, KC; Dravid, VW. Interaction of fatty acid monolayers with cobalt nanoparticles. Nano Lett 2004, 4, 383–386. [Google Scholar]
- Song, HT; Huh, YM; Kim, S; Jun, WW; Suh, JS; Cheon, J. Surface modulation of magnetic nanocrystals in the development of highly efficient magnetic resonance probes for intracellular labeling. J. Am. Chem. Soc 2005, 127, 9992–9993. [Google Scholar]
- da Silva, EC; da Silva, MGA; Meneghetti, SMP; Machado, G; Alencar, MARC; Hickmann, JM; Meneghetti, MR. Synthesis of colloids based on gold nanoparticles dispersed in castor oil. J. Nanopart. Res 2008, 10, 201–208. [Google Scholar]
- Xu, B; Xiao, G; Cui, L; Wei, R; Gao, L. Transesterification of Palm Oil with Methanol to Biodiesel over a KF/Al2O3 Heterogeneous Base Catalyst. Energy Fuels 2007, 21, 3109–3112. [Google Scholar]
- Pyatenko, A; Yamaguchi, M; Suzuki, M. Laser photolysis of silver colloid prepared by citric acid reduction method. J. Phys. Chem. B 2005, 109, 21608–21611. [Google Scholar]
- Mafune, F; Kohno, J; Takeda, Y; Kondow, T. Formation and Size Control of Silver Nanoparticles by Laser Ablation in Aqueous Solution. J. Phys. Chem. B 2000, 104, 9111–9117. [Google Scholar]
- Qiu, L; Liu, F; Zhao, L; Yang, W; Yao, J. Evidence of a Unique Electron Donor-Acceptor Property for Platinum Nanoparticles as Studied by XPS. Langmuir 2006, 22, 4480–4482. [Google Scholar]
- Roucoux, AI; Schulz, J; Henri, P. Reduced Transition Metal Colloids: A Novel Family of Reusable Catalysts. Chem. Rev 2002, 102, 3757–3778. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zamiri, R.; Zakaria, A.; Ahangar, H.A.; Sadrolhosseini, A.R.; Mahdi, M.A. Fabrication of Silver Nanoparticles Dispersed in Palm Oil Using Laser Ablation. Int. J. Mol. Sci. 2010, 11, 4764-4770. https://doi.org/10.3390/ijms11114764
Zamiri R, Zakaria A, Ahangar HA, Sadrolhosseini AR, Mahdi MA. Fabrication of Silver Nanoparticles Dispersed in Palm Oil Using Laser Ablation. International Journal of Molecular Sciences. 2010; 11(11):4764-4770. https://doi.org/10.3390/ijms11114764
Chicago/Turabian StyleZamiri, Reza, Azmi Zakaria, Hossein Abbastabar Ahangar, Amir Reza Sadrolhosseini, and Mohd Adzir Mahdi. 2010. "Fabrication of Silver Nanoparticles Dispersed in Palm Oil Using Laser Ablation" International Journal of Molecular Sciences 11, no. 11: 4764-4770. https://doi.org/10.3390/ijms11114764
APA StyleZamiri, R., Zakaria, A., Ahangar, H. A., Sadrolhosseini, A. R., & Mahdi, M. A. (2010). Fabrication of Silver Nanoparticles Dispersed in Palm Oil Using Laser Ablation. International Journal of Molecular Sciences, 11(11), 4764-4770. https://doi.org/10.3390/ijms11114764