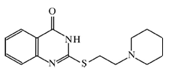
A Novel PARP Inhibitor L-2286 in a Rat Model of Impact Acceleration Head Injury: An Immunohistochemical and Behavioral Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dose-Response Curve of i.c.v. L-2286 after Impact Acceleration Injury
2.2. Results of Immunohistochemical Analysis
2.3. Effect of 100 μg/rat of i.c.v. L-2286 on Beam-Balance Test Performance after IA
2.4. Effect of 100 μg/rat of i.c.v. L-2286 on Open-Field Performance after IA
2.5. Effect of 100 μg/rat of i.c.v. L-2286 on Elevated Plus-Maze Performance after IA
2.6. Discussion
3. Experimental Section
3.1. Injury Induction
3.2. Establishment of Dose-Response Curve for i.c.v. L-2286
3.3. Experimental Protocol to Test the Post-Injury Administration Regime
3.4. Immunohistochemistry
3.5. Image Analysis
3.6. Behavioral Tests
3.6.1. Beam-Balance Test
3.6.2. Open-Field Test
3.6.3. Elevated Plus-Maze Test
3.6.4. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Susin, SA; Lorenzo, HK; Zamzami, N; Marzo, I; Snow, BE; Brothers, GM. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar]
- Du, L; Zhang, X; Han, YY; Burke, NA; Kochanek, PM; Watkins, SC. Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J. Biol. Chem 2003, 278, 18426–18433. [Google Scholar]
- Zhang, X; Chen, J; Graham, SH; Du, L; Kochanek, PM; Draviam, R. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J. Neurochem 2002, 82, 181–191. [Google Scholar]
- Cao, G; Clark, RS; Pei, W; Yin, W; Zhang, F; Sun, FY. Translocation of apoptosis-inducing factor in vulnerable neurons after transient cerebral ischemia and in neuronal cultures after oxygen-glucose deprivation. J. Cereb. Blood Flow Metab 2003, 23, 1137–1150. [Google Scholar]
- Satchell, MA; Zhang, X; Kochanek, PM; Dixon, CE; Jenkins, LW; Melick, J. A dual role for poly-ADP-ribosylation in spatial memory acquisition after traumatic brain injury in mice involving NAD+ depletion and ribosylation of 14-3-3gamma. J. Neurochem 2003, 85, 697–708. [Google Scholar]
- LaPlaca, MC; Zhang, J; Raghupathi, R; Li, JH; Smith, F; Bareyre, FM. Pharmacologic inhibition of poly(ADP-ribose) polymerase is neuroprotective following traumatic brain injury in rats. J. Neurotrauma 2001, 18, 369–376. [Google Scholar]
- Besson, VC; Croci, N; Boulu, RG; Plotkine, M; Marchand-Verrecchia, C. Deleterious poly(ADP-ribose)polymerase-1 pathway activation in traumatic brain injury in rat. Brain Res 2003, 989, 58–66. [Google Scholar]
- Besson, VC; Zsengeller, Z; Plotkine, M; Szabo, C; Marchand-Verrecchia, C. Beneficial effects of PJ34 and INO-1001, two novel water-soluble poly(ADP-ribose) polymerase inhibitors, on the consequences of traumatic brain injury in rat. Brain Res 2005, 1041, 149–156. [Google Scholar]
- Palfi, A; Toth, A; Kulcsar, G; Hanto, K; Deres, P; Bartha, E. The role of Akt and mitogen-activated protein kinase systems in the protective effect of poly(ADP-ribose) polymerase inhibition in Langendorff perfused and in isoproterenol-damaged rat hearts. J. Pharmacol. Exp. Ther 2005, 315, 273–282. [Google Scholar]
- Clark, RS; Vagni, VA; Nathaniel, PD; Jenkins, LW; Dixon, CE; Szabo, C. Local administration of the poly(ADP-ribose) polymerase inhibitor INO-1001 prevents NAD+ depletion and improves water maze performance after traumatic brain injury in mice. J. Neurotrauma 2007, 24, 1399–1405. [Google Scholar]
- Marmarou, CR; Walker, SA; Davis, CL; Povlishock, JT. Quantitative analysis of the relationship between intra- axonal neurofilament compaction and impaired axonal transport following diffuse traumatic brain injury. J. Neurotrauma 2005, 22, 1066–1080. [Google Scholar]
- Stone, JR; Singleton, RH; Povlishock, JT. Intra-axonal neurofilament compaction does not evoke local axonal swelling in all traumatically injured axons. Exp. Neurol 2001, 172, 320–331. [Google Scholar]
- Heath, DL; Vink, R. Optimization of magnesium therapy after severe diffuse axonal brain injury in rats. J. Pharmacol. Exp. Ther 1999, 288, 1311–1316. [Google Scholar]
- Schmidt, RH; Scholten, KJ; Maughan, PH. Cognitive impairment and synaptosomal choline uptake in rats following impact acceleration injury. J. Neurotrauma 2000, 17, 1129–1139. [Google Scholar]
- Vink, R; O’Connor, CA; Nimmo, AJ; Heath, DL. Magnesium attenuates persistent functional deficits following diffuse traumatic brain injury in rats. Neurosci. Lett 2003, 336, 41–44. [Google Scholar]
- Beaumont, A; Marmarou, A; Czigner, A; Yamamoto, M; Demetriadou, K; Shirotani, T. The impact-acceleration model of head injury: injury severity predicts motor and cognitive performance after trauma. Neurol. Res 1999, 21, 742–754. [Google Scholar]
- Adelson, PD; Dixon, CE; Robichaud, P; Kochanek, PM. Motor and cognitive functional deficits following diffuse traumatic brain injury in the immature rat. J. Neurotrauma 1997, 14, 99–108. [Google Scholar]
- Berman, RF; Verweij, BH; Muizelaar, JP. Neurobehavioral protection by the neuronal calcium channel blocker ziconotide in a model of traumatic diffuse brain injury in rats. J. Neurosurg 2000, 93, 821–828. [Google Scholar]
- Coyle, JT; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar]
- Facchinetti, F; Dawson, VL; Dawson, TM. Free radicals as mediators of neuronal injury. Cell Mol. Neurobiol 1998, 18, 667–682. [Google Scholar]
- Iwashita, A; Tojo, N; Matsuura, S; Yamazaki, S; Kamijo, K; Ishida, J. A novel and potent poly(ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3,6-dihydro-1(2H)-pyridinyl)propyl]-4(3H)-quinazo linone), attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia. J. Pharmacol. Exp. Ther 2004, 310, 425–436. [Google Scholar]
- Takahashi, K; Greenberg, JH; Jackson, P; Maclin, K; Zhang, J. Neuroprotective effects of inhibiting poly (ADP-ribose) synthetase on focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab 1997, 17, 1137–1142. [Google Scholar]
- Takahashi, K; Pieper, AA; Croul, SE; Zhang, J; Snyder, SH; Greenberg, JH. Post-treatment with an inhibitor of poly (ADP-ribose) polymerase attenuates cerebral damage in focal ischemia. Brain Res 1999, 829, 46–54. [Google Scholar]
- Takahashi, K; Greenberg, JH. The effect of reperfusion on neuroprotection using an inhibitor of poly(ADP-ribose) polymerase. Neuroreport 1999, 10, 2017–2022. [Google Scholar]
- Endres, M; Wang, ZQ; Namura, S; Waeber, C; Moskowitz, MA. Ischemic brain injury is mediated by the activation of poly(ADP-ribose)polymerase. J. Cereb. Blood Flow Metab 1997, 17, 1143–1151. [Google Scholar]
- Zhang, J; Li, JH; Lautar, S. Post-ischemia protection by a potent PARP inhibitor in transient cerebral focal ischemia. Abstr. Soc. Neurosci 1998, 24, 1226. [Google Scholar]
- Ding, Y; Zhou, Y; Lai, Q; Li, J; Gordon, V; Diaz, FG. Long-term neuroprotective effect of inhibiting poly(ADP-ribose) polymerase in rats with middle cerebral artery occlusion using a behavioral assessment. Brain Res 2001, 915, 210–217. [Google Scholar]
- Ilnytska, O; Lyzogubov, VV; Stevens, MJ; Drel, VR; Mashtalir, N; Pacher, P. Poly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes 2006, 55, 1686–1694. [Google Scholar]
- Suh, SW; Aoyama, K; Chen, Y; Garnier, P; Matsumori, Y; Gum, E. Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J. Neurosci 2003, 23, 10681–10690. [Google Scholar]
- Buki, A; Okonkwo, DO; Wang, KK; Povlishock, JT. Cytochrome c release and caspase activation in traumatic axonal injury. J. Neurosci 2000, 20, 2825–2834. [Google Scholar]
- Koizumi, H; Povlishock, JT. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J. Neurosurg 1998, 89, 303–309. [Google Scholar]
- Okonkwo, DO; Buki, A; Siman, R; Povlishock, JT. Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. Neuroreport 1999, 10, 353–358. [Google Scholar]
- Buki, A; Okonkwo, DO; Povlishock, JT. Postinjury cyclosporin A administration limits axonal damage and disconnection in traumatic brain injury. J. Neurotrauma 1999, 16, 511–521. [Google Scholar]
- Blumbergs, PC; Scott, G; Manavis, J; Wainwright, H; Simpson, DA; McLean, AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet 1994, 344, 1055–1056. [Google Scholar]
- Bramlett, HM; Kraydieh, S; Green, EJ; Dietrich, WD. Temporal and regional patterns of axonal damage following traumatic brain injury: A beta-amyloid precursor protein immunocytochemical study in rats. J. Neuropathol. Exp. Neurol 1997, 56, 1132–1141. [Google Scholar]
- Buki, A; Farkas, O; Doczi, T; Povlishock, JT. Preinjury administration of the calpain inhibitor MDL-28170 attenuates traumatically induced axonal injury. J. Neurotrauma 2003, 20, 261–268. [Google Scholar]
- Gentleman, SM; Nash, MJ; Sweeting, CJ; Graham, DI; Roberts, GW. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci. Lett 1993, 160, 139–144. [Google Scholar]
- Stone, JR; Singleton, RH; Povlishock, JT. Antibodies to the C-terminus of the beta-amyloid precursor protein (APP): A site specific marker for the detection of traumatic axonal injury. Brain Res 2000, 871, 288–302. [Google Scholar]
- Buki, A; Koizumi, H; Povlishock, JT. Moderate posttraumatic hypothermia decreases early calpain-mediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury. Exp. Neurol 1999, 159, 319–328. [Google Scholar]
- Buki, A; Siman, R; Trojanowski, JQ; Povlishock, JT. The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J. Neuropathol Exp. Neurol 1999, 58, 365–375. [Google Scholar]
- Okonkwo, DO; Pettus, EH; Moroi, J; Povlishock, JT. Alteration of the neurofilament sidearm and its relation to neurofilament compaction occurring with traumatic axonal injury. Brain Res 1998, 784, 1–6. [Google Scholar]
- Povlishock, JT; Pettus, EH. Traumatically induced axonal damage: Evidence for enduring changes in axolemmal permeability with associated cytoskeletal change. Acta Neurochir. Suppl 1996, 66, 81–86. [Google Scholar]
- Povlishock, JT; Marmarou, A; McIntosh, T; Trojanowski, JQ; Moroi, J. Impact acceleration injury in the rat: Evidence for focal axolemmal change and related neurofilament sidearm alteration. J. Neuropathol. Exp. Neurol 1997, 56, 347–359. [Google Scholar]
- Stone, JR; Walker, SA; Povlishock, JT. The visualization of a new class of traumatically injured axons through the use of a modified method of microwave antigen retrieval. Acta Neuropathol 1999, 97, 335–345. [Google Scholar]
- Clifton, GL; Jiang, JY; Lyeth, BG; Jenkins, LW; Hamm, RJ; Hayes, RL. Marked protection by moderate hypothermia after experimental traumatic brain injury. J. Cereb. Blood Flow. Metab 1991, 11, 114–121. [Google Scholar]










© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kövesdi, E.; Bukovics, P.; Besson, V.; Nyirádi, J.; Lückl, J.; Pál, J.; Sümegi, B.; Dóczi, T.; Hernádi, I.; Büki, A. A Novel PARP Inhibitor L-2286 in a Rat Model of Impact Acceleration Head Injury: An Immunohistochemical and Behavioral Study. Int. J. Mol. Sci. 2010, 11, 1253-1268. https://doi.org/10.3390/ijms11041253
Kövesdi E, Bukovics P, Besson V, Nyirádi J, Lückl J, Pál J, Sümegi B, Dóczi T, Hernádi I, Büki A. A Novel PARP Inhibitor L-2286 in a Rat Model of Impact Acceleration Head Injury: An Immunohistochemical and Behavioral Study. International Journal of Molecular Sciences. 2010; 11(4):1253-1268. https://doi.org/10.3390/ijms11041253
Chicago/Turabian StyleKövesdi, Erzsébet, Péter Bukovics, Valérie Besson, József Nyirádi, János Lückl, József Pál, Balázs Sümegi, Tamás Dóczi, István Hernádi, and András Büki. 2010. "A Novel PARP Inhibitor L-2286 in a Rat Model of Impact Acceleration Head Injury: An Immunohistochemical and Behavioral Study" International Journal of Molecular Sciences 11, no. 4: 1253-1268. https://doi.org/10.3390/ijms11041253