Mechanobiology of Platelets: Techniques to Study the Role of Fluid Flow and Platelet Retraction Forces at the Micro- and Nano-Scale
Abstract
:1. Introduction
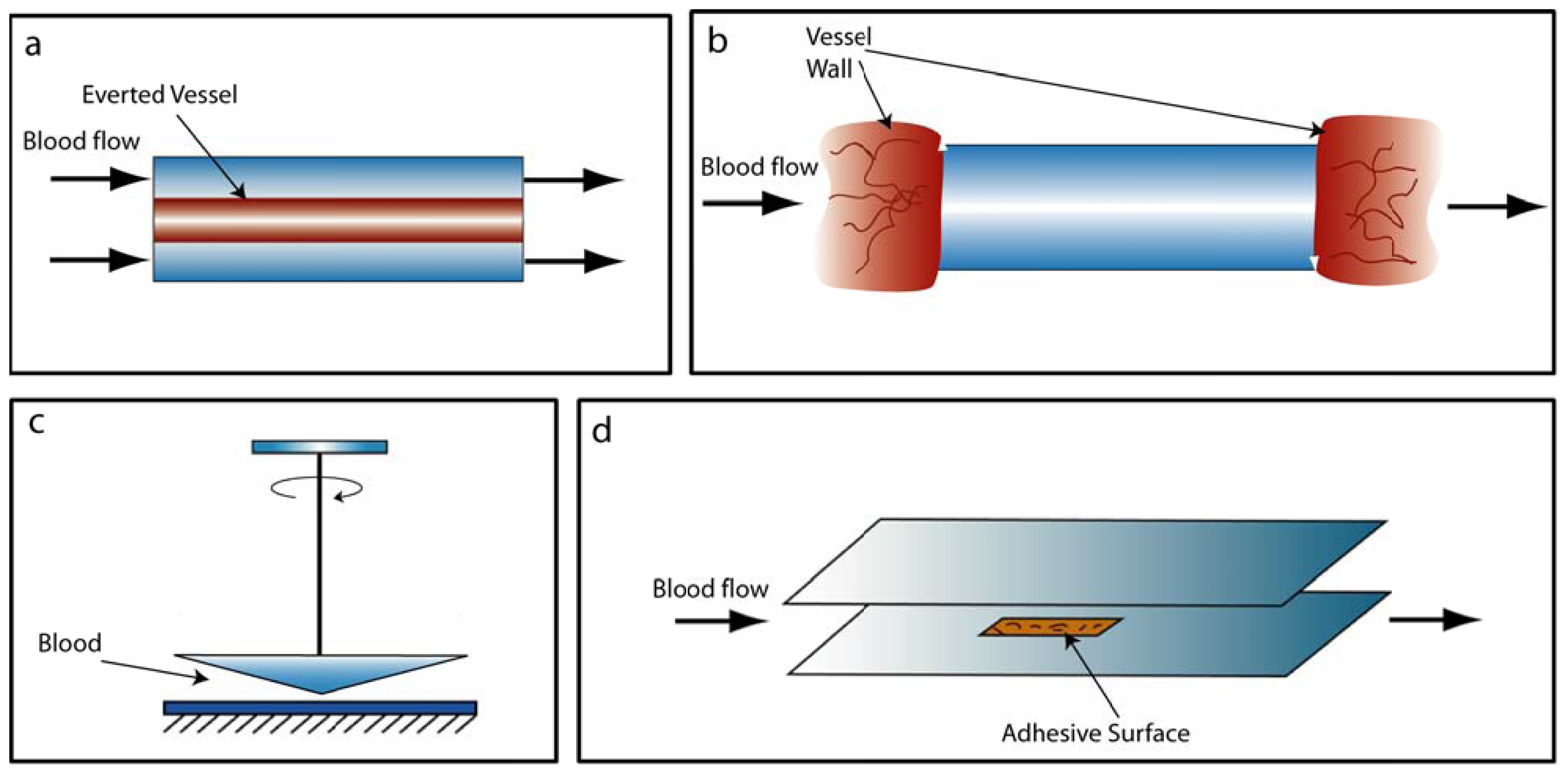
2. Platelet-Shear Flow Interactions
2.1. Conventional Devices
2.2. Microfluidic Devices
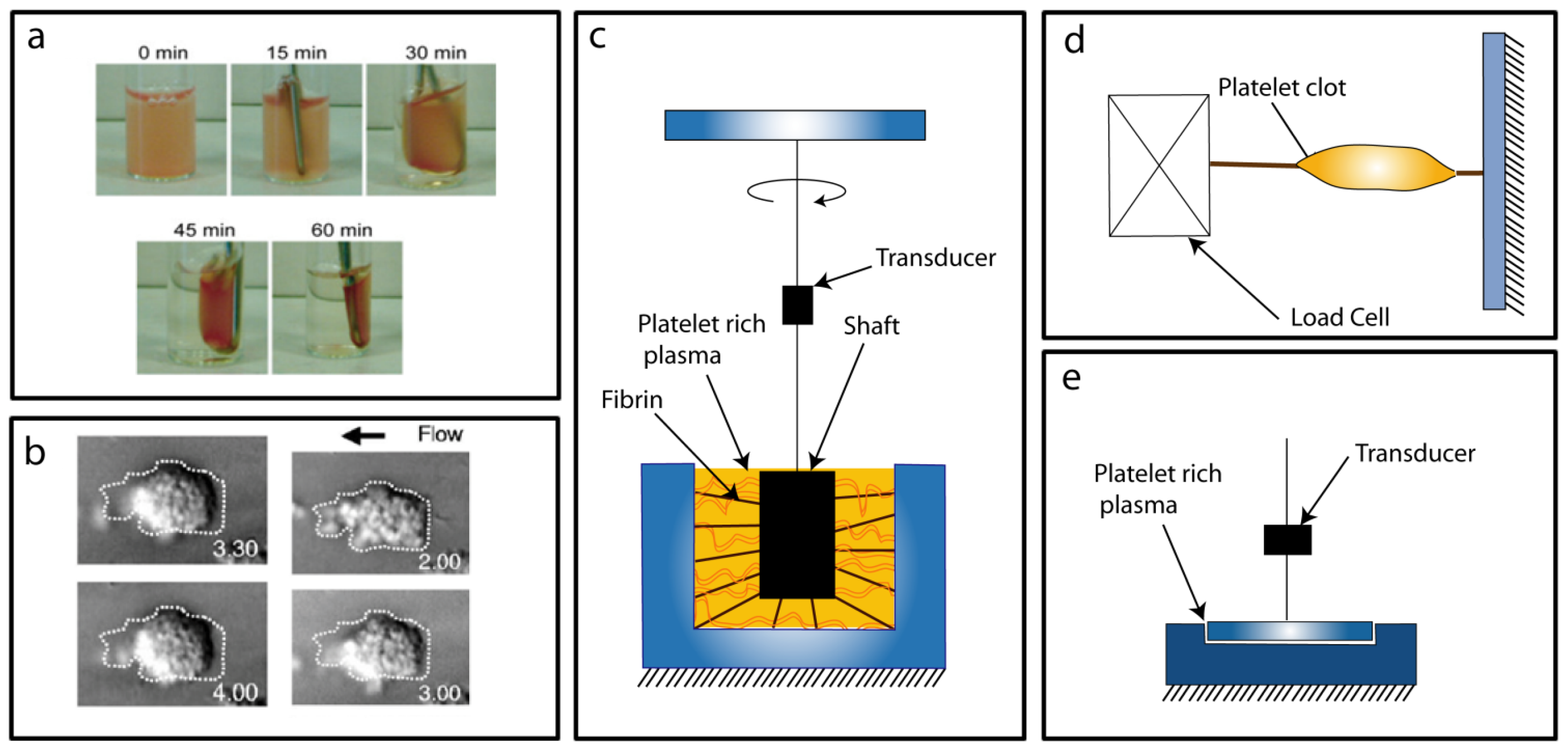
3. Platelet Clot Retraction
3.1. Conventional Force Assays
3.2. Micro/Nano Force Assays
4. Conclusion and Future Directions
Acknowledgments
References
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg 1998, 176, 26S–38S. [Google Scholar]
- Michelson, A.D. Platelets, 2nd ed; Academic Press/Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Weiss, H.J. Platelet physiology and abnormalities of platelet function (first of two parts). N. Engl. J. Med 1975, 293, 531–541. [Google Scholar]
- Weiss, H.J. Platelet physiology and abnormalities of platelet function (second of two parts). N. Engl. J. Med 1975, 293, 580–588. [Google Scholar]
- Maxwell, M.J.; Westein, E.; Nesbitt, W.S.; Giuliano, S.; Dopheide, S.M.; Jackson, S.P. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood 2007, 109, 566–576. [Google Scholar]
- Kuwahara, M.; Sugimoto, M.; Tsuji, S.; Matsui, H.; Mizuno, T.; Miyata, S.; Yoshioka, A. Platelet shape changes and adhesion under high shear flow. Arterioscler. Thromb. Vasc. Biol 2002, 22, 329–334. [Google Scholar]
- Maxwell, M.J.; Dopheide, S.M.; Turner, S.J.; Jackson, S.P. Shear induces a unique series of morphological changes in translocating platelets: Effects of morphology on translocation dynamics. Arterioscler. Thromb. Vasc. Biol 2006, 26, 663–669. [Google Scholar]
- Sixma, J.J.; Wester, J. The hemostatic plug. Semin Hematol 1977, 14, 265–299. [Google Scholar]
- Sixma, J.J.; de Groot, P.G. Regulation of platelet adhesion to the vessel wall. Ann. N. Y. Acad. Sci 1994, 714, 190–199. [Google Scholar]
- Ruggeri, Z.M.; Dent, J.A.; Saldivar, E. Contribution of distinct adhesive interactions to platelet aggregation in flowing blood. Blood 1999, 94, 172–178. [Google Scholar]
- Savage, B.; Saldivar, E.; Ruggeri, Z.M. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 1996, 84, 289–297. [Google Scholar]
- Tsuji, S.; Sugimoto, M.; Miyata, S.; Kuwahara, M.; Kinoshita, S.; Yoshioka, A. Real-time analysis of mural thrombus formation in various platelet aggregation disorders: Distinct shear-dependent roles of platelet receptors and adhesive proteins under flow. Blood 1999, 94, 968–975. [Google Scholar]
- Jackson, S.P. The growing complexity of platelet aggregation. Blood 2007, 109, 5087–5095. [Google Scholar]
- Frenette, P.S.; Johnson, R.C.; Hynes, R.O.; Wagner, D.D. Platelets roll on stimulated endothelium in vivo: An interaction mediated by endothelial P-selectin. Proc. Natl. Acad. Sci. USA 1995, 92, 7450–7454. [Google Scholar]
- Frenette, P.S.; Denis, C.V.; Weiss, L.; Jurk, K.; Subbarao, S.; Kehrel, B.; Hartwig, J.H.; Vestweber, D.; Wagner, D.D. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J. Exp. Med 2000, 191, 1413–1422. [Google Scholar]
- Chen, J.; Lopez, J.A. Interactions of platelets with subendothelium and endothelium. Microcirculation 2005, 12, 235–246. [Google Scholar]
- Andrews, R.K.; Berndt, M.C. Platelet physiology and thrombosis. Thromb. Res 2004, 114, 447–453. [Google Scholar]
- Brass, L.F.; Zhu, L.; Stalker, T.J. Minding the gaps to promote thrombus growth and stability. J. Clin. Invest 2005, 115, 3385–3392. [Google Scholar]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between the extracellular matrix—Cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol 2001, 2, 793–805. [Google Scholar]
- Ruggeri, Z.M. Mechanisms initiating platelet thrombus formation. Thromb. Haemost 1997, 78, 611–616. [Google Scholar]
- Ruggeri, Z.M.; Orje, J.N.; Habermann, R.; Federici, A.B.; Reininger, A.J. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 2006, 108, 1903–1910. [Google Scholar]
- Savage, B.; Almus-Jacobs, F.; Ruggeri, Z.M. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell 1998, 94, 657–666. [Google Scholar]
- Dopheide, S.M.; Maxwell, M.J.; Jackson, S.P. Shear-dependent tether formation during platelet translocation on von Willebrand factor. Blood 2002, 99, 159–167. [Google Scholar]
- Nesbitt, W.S.; Westein, E.; Tovar-Lopez, F.J.; Tolouei, E.; Mitchell, A.; Fu, J.; Carberry, J.; Fouras, A.; Jackson, S.P. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat. Med 2009, 15, 665–673. [Google Scholar]
- Ono, A.; Westein, E.; Hsiao, S.; Nesbitt, W.S.; Hamilton, J.R.; Schoenwaelder, S.M.; Jackson, S.P. Identification of a fibrin-independent platelet contractile mechanism regulating primary hemostasis and thrombus growth. Blood 2008, 112, 90–99. [Google Scholar]
- Liang, X.M.; Han, S.J.; Reems, J.A.; Gao, D.Y.; Sniadecki, N.J. Platelet retraction force measurements using flexible post force sensors. Lab Chip 2010, 10, 991–998. [Google Scholar]
- Lam, W.A.; Chaudhuri, O.; Crow, A.; Webster, K.D.; Li, T.D.; Kita, A.; Huang, J.; Fletcher, D.A. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat. Mater 2011, 10, 61–66. [Google Scholar]
- Koteliansky, V.E.; Leytin, V.L.; Sviridov, D.D.; Repin, V.S.; Smirnov, V.N. Human plasma fibronectin promotes the adhesion and spreading of platelets on surfaces coated with fibrillar collagen. FEBS Lett 1981, 123, 59–62. [Google Scholar]
- Leytin, V.L.; Ljubimova, E.V.; Sviridov, D.D.; Repin, V.S.; Smirnov, V.N. Time-response changes in the thrombogenicity of platelets spread on a collagen-coated surface. Thromb. Res 1980, 20, 335–341. [Google Scholar]
- Frojmovic, M.; Longmire, K.; van de Ven, T.G. Long-range interactions in mammalian platelet aggregation. II. The role of platelet pseudopod number and length. Biophys. J 1990, 58, 309–318. [Google Scholar]
- Feuerstein, I.A.; Ratner, B.D. Adhesion and aggregation of thrombin prestimulated human platelets: Evaluation of a series of biomaterials characterized by ESCA. Biomaterials 1990, 11, 127–132. [Google Scholar]
- Eckstein, E.C.; Bilsker, D.L.; Waters, C.M.; Kippenhan, J.S.; Tilles, A.W. Transport of platelets in flowing blood. Ann. N. Y. Acad. Sci 1987, 516, 442–452. [Google Scholar]
- Yeh, C.; Eckstein, E.C. Transient lateral transport of platelet-sized particles in flowing blood suspensions. Biophys. J 1994, 66, 1706–1716. [Google Scholar]
- Spijker, H.T.; Graaff, R.; Boonstra, P.W.; Busscher, H.J.; van Oeveren, W. On the influence of flow conditions and wettability on blood material interactions. Biomaterials 2003, 24, 4717–4727. [Google Scholar]
- Jackson, S.P.; Nesbitt, W.S.; Westein, E. Dynamics of platelet thrombus formation. J. Thromb. Haemost. 2009, 7(Suppl 1), 17–20. [Google Scholar]
- de Groot, P.G.; IJsseldijk, M.J.W.; Sixma, J.J. Platelet adhesion to the subendothelium under flow. Methods Mol. Biol 1999, 96, 159–170. [Google Scholar]
- Baumgartner, H.R.; Haudenschild, C. Adhesion of platelets to subendothelium. Ann. N. Y. Acad. Sci 1972, 201, 22–36. [Google Scholar]
- Anderson, G.H.; Hellums, J.D.; Moake, J.; Alfrey, C.P., Jr. Platelet response to shear stress: Changes in serotonin uptake, serotonin release, and ADP induced aggregation. Thromb. Res 1978, 13, 1039–1047. [Google Scholar]
- Weiss, H.J.; Turitto, V.T.; Baumgartner, H.R. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. I. Shear rate—Dependent decrease of adhesion in von Willebrand’s disease and the Bernard-Soulier syndrome. J. Lab. Clin. Med 1978, 92, 750–764. [Google Scholar]
- Tschopp, T.B.; Weiss, H.J.; Baumgartner, H.R. Decreased adhesion of platelets to subendothelium in von Willebrand’s disease. J. Lab. Clin. Med 1974, 83, 296–300. [Google Scholar]
- Weiss, H.J.; Tschopp, T.B.; Baumgartner, H.R.; Sussman, I.I.; Johnson, M.M.; Egan, J.J. Decreased adhesion of giant (Bernard-Soulier) platelets to subendothelium. Further implications on the role of the von Willebrand factor in hemostasis. Am. J. Med 1974, 57, 920–925. [Google Scholar]
- Weiss, H.J.; Tschopp, T.B.; Baumgartner, H.R. Impaired interaction (adhesion-aggregation) of platelets with the subendothelium in storage-pool disease and after aspirin ingestion. A comparison with von Willebrand's disease. N. Engl. J. Med 1975, 293, 619–623. [Google Scholar]
- Sakariassen, K.S.; Nievelstein, P.F.; Coller, B.S.; Sixma, J.J. The role of platelet membrane glycoproteins Ib and IIb-IIIa in platelet adherence to human artery subendothelium. Br. J. Haematol 1986, 63, 681–691. [Google Scholar]
- Badimon, L.; Turitto, V.; Rosemark, J.A.; Badimon, J.J.; Fuster, V. Characterization of a tubular flow chamber for studying platelet interaction with biologic and prosthetic materials: Deposition of indium 111-labeled platelets on collagen, subendothelium, and expanded polytetrafluoroethylene. J. Lab. Clin. Med 1987, 110, 706–718. [Google Scholar]
- Roald, H.E.; Barstad, R.M.; Bakken, I.J.; Roald, B.; Lyberg, T.; Sakariassen, K.S. Initial interactions of platelets and plasma proteins in flowing non-anticoagulated human blood with the artificial surfaces Dacron and PTFE. Blood Coagul. Fibrinolysis 1994, 5, 355–363. [Google Scholar]
- Chen, C.; Ofenloch, J.C.; Yianni, Y.P.; Hanson, S.R.; Lumsden, A.B. Phosphorylcholine coating of ePTFE reduces platelet deposition and neointimal hyperplasia in arteriovenous grafts. J. Surg. Res 1998, 77, 119–125. [Google Scholar]
- Hanson, S.R.; Kotze, H.F.; Savage, B.; Harker, L.A. Platelet interactions with Dacron vascular grafts. A model of acute thrombosis in baboons. Arteriosclerosis 1985, 5, 595–603. [Google Scholar]
- Cheng, H.; Yan, R.; Li, S.; Yuan, Y.; Liu, J.; Ruan, C.; Dai, K. Shear-induced interaction of platelets with von Willebrand factor results in glycoprotein Ibalpha shedding. Am. J. Physiol. Heart Circ. Physiol 2009, 297, H2128–H2135. [Google Scholar]
- Brown, C.H., III; Leverett, L.B.; Lewis, C.W.; Alfrey, C.P., Jr.; Hellums,, J.D. Morphological, biochemical, and functional changes in human platelets subjected to shear stress. J. Lab. Clin. Med 1975, 86, 462–471. [Google Scholar]
- Giorgio, T.D.; Hellums, J.D. A cone and plate viscometer for the continuous measurement of blood platelet activation. Biorheology 1988, 25, 605–624. [Google Scholar]
- Furukawa, K.; Ushida, T.; Sugano, H.; Ohshima, N.; Tateishi, T. Real time observation of platelet adhesion to opaque biomaterial surfaces under shear flow conditions. J. Biomed. Mater. Res 1999, 46, 93–102. [Google Scholar]
- Chow, T.W.; Hellums, J.D.; Moake, J.L.; Kroll, M.H. Shear stress-induced von Willebrand factor binding to platelet glycoprotein Ib initiates calcium influx associated with aggregation. Blood 1992, 80, 113–120. [Google Scholar]
- Panzer, S.; Badr Eslam, R.; Schneller, A.; Kaider, A.; Koren, D.; Eichelberger, B.; Rosenhek, R.; Budde, U.; Lang, I.M. Loss of high-molecular-weight von Willebrand factor multimers mainly affects platelet aggregation in patients with aortic stenosis. Thromb. Haemost 2010, 103, 408–414. [Google Scholar]
- Yin, W.; Shanmugavelayudam, S.K.; Rubenstein, D.A. The effect of physiologically relevant dynamic shear stress on platelet and endothelial cell activation. Thromb. Res 2011, 127, 235–241. [Google Scholar]
- Furukawa, K.S.; Nakamura, K.; Onimura, Y.; Uchida, M.; Ito, A.; Yamane, T.; Tamaki, T.; Ushida, T.; Tateishi, T. Quantitative analysis of human platelet adhesions under a small-scale flow device. Artif. Organs 2010, 34, 295–300. [Google Scholar]
- Sakariassen, K.S.; Aarts, P.A.; de Groot, P.G.; Houdijk, W.P.; Sixma, J.J. A perfusion chamber developed to investigate platelet interaction in flowing blood with human vessel wall cells, their extracellular matrix, and purified components. J. Lab. Clin. Med 1983, 102, 522–535. [Google Scholar]
- Hubbell, J.A.; McIntire, L.V. Visualization and analysis of mural thrombogenesis on collagen, polyurethane and nylon. Biomaterials 1986, 7, 354–363. [Google Scholar]
- Sakariassen, K.S.; Kuhn, H.; Muggli, R.; Baumgartner, H.R. Growth and stability of thrombi in flowing citrated blood: Assessment of platelet-surface interactions with computer-assisted morphometry. Thromb. Haemost 1988, 60, 392–398. [Google Scholar]
- van Breugel, H.H.; Sixma, J.J.; Heethaar, R.M. Effects of flow pulsatility on platelet adhesion to subendothelium. Arteriosclerosis 1988, 8, 332–335. [Google Scholar]
- Barstad, R.M.; Kierulf, P.; Sakariassen, K.S. Collagen induced thrombus formation at the apex of eccentric stenoses—A time course study with non-anticoagulated human blood. Thromb. Haemost 1996, 75, 685–692. [Google Scholar]
- de Graaf, J.C.; Banga, J.D.; Moncada, S.; Palmer, R.M.; de Groot, P.G.; Sixma, J.J. Nitric oxide functions as an inhibitor of platelet adhesion under flow conditions. Circulation 1992, 85, 2284–2290. [Google Scholar]
- Roux, S.P.; Sakariassen, K.S.; Turitto, V.T.; Baumgartner, H.R. Effect of aspirin and epinephrine on experimentally induced thrombogenesis in dogs. A parallelism between in vivo and ex vivo thrombosis models. Arterioscler. Thromb 1991, 11, 1182–1191. [Google Scholar]
- Sakariassen, K.S.; Bolhuis, P.A.; Sixma, J.J. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature 1979, 279, 636–638. [Google Scholar]
- Ruggeri, Z.M.; Mendolicchio, G.L. Adhesion mechanisms in platelet function. Circ. Res 2007, 100, 1673–1085. [Google Scholar]
- Kirchhofer, D.; Tschopp, T.B.; Baumgartner, H.R. Active site-blocked factors VIIa and IXa differentially inhibit fibrin formation in a human ex vivo thrombosis model. Arterioscler. Thromb. Vasc. Biol 1995, 15, 1098–1106. [Google Scholar]
- Sixma, J.J.; de Groot, P.G.; van Zanten, H.; IJsseldijk, M. A new perfusion chamber to detect platelet adhesion using a small volume of blood. Thromb. Res 1998, 92, S43–S46. [Google Scholar]
- Xia, Y.N.; Whitesides, G.M. Soft lithography. Angew. Chem. Int. Ed 1998, 37, 551–575. [Google Scholar]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem 1998, 70, 4974–4984. [Google Scholar]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng 2001, 3, 335–373. [Google Scholar]
- van der Meer, A.D.; Poot, A.A.; Duits, M.H.G.; Feijen, J.; Vermes, I. Microfluidic technology in vascular research. J. Biomed. Biotechnol 2009, 2009. [Google Scholar] [CrossRef]
- Prabhakarpandian, B.; Shen, M.C.; Pant, K.; Kiani, M.F. Microfluidic devices for modeling cell-cell and particle-cell interactions in the microvasculature. Microvasc. Res 2011, 82, 210–220. [Google Scholar]
- Remijn, J.A.; Wu, Y.P.; Ijsseldijk, M.J.; Zwaginga, J.J.; Sixma, J.J.; de Groot, P.G. Absence of fibrinogen in afibrinogenemia results in large but loosely packed thrombi under flow conditions. Thromb. Haemost 2001, 85, 736–742. [Google Scholar]
- Neeves, K.B.; Diamond, S.L. A membrane-based microfluidic device for controlling the flux of platelet agonists into flowing blood. Lab Chip 2008, 8, 701–709. [Google Scholar]
- Conant, C.G.; Nevill, J.T.; Zhou, Z.; Dong, J.F.; Schwartz, M.A.; Ionescu-Zanetti, C. Using well-plate microfluidic devices to conduct shear-based thrombosis assays. J. Lab. Autom 2011, 16, 148–152. [Google Scholar]
- Tovar-Lopez, F.J.; Rosengarten, G.; Westein, E.; Khoshmanesh, K.; Jackson, S.P.; Mitchell, A.; Nesbitt, W.S. A microfluidics device to monitor platelet aggregation dynamics in response to strain rate micro-gradients in flowing blood. Lab Chip 2010, 10, 291–302. [Google Scholar]
- Hoff, J. Methods of blood collection in the mouse. Lab. Anim 2000, 29, 47–53. [Google Scholar]
- Neeves, K.B.; Maloney, S.F.; Fong, K.P.; Schmaier, A.A.; Kahn, M.L.; Brass, L.F.; Diamond, S.L. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. J. Thromb. Haemost 2008, 6, 2193–2201. [Google Scholar]
- Kent, N.J.; O’Brien, S.; Basabe-Desmonts, L.; Meade, G.R.; MacCraith, B.D.; Corcoran, B.G.; Kenny, D.; Ricco, A.J. Shear-mediated platelet adhesion analysis in less than 100 μL of blood: Toward a POC platelet diagnostic. IEEE Trans. Biomed. Eng 2011, 58, 826–830. [Google Scholar]
- Stolla, M.; Stefanini, L.; Roden, R.C.; Chavez, M.; Hirsch, J.; Greene, T.; Ouellette, T.D.; Maloney, S.F.; Diamond, S.L.; Poncz, M.; et al. The kinetics of αIIbβ3 activation determines the size and stability of thrombi in mice: Implications for antiplatelet therapy. Blood 2011, 117, 1005–1013. [Google Scholar]
- Wannemacher, K.M.; Zhu, L.; Jiang, H.; Fong, K.P.; Stalker, T.J.; Lee, D.; Tran, A.N.; Neeves, K.B.; Maloney, S.; Kumanogoh, A.; et al. Diminished contact-dependent reinforcement of Syk activation underlies impaired thrombus growth in mice lacking Semaphorin 4D. Blood 2010, 116, 5707–5715. [Google Scholar]
- Gutierrez, E.; Petrich, B.G.; Shattil, S.J.; Ginsberg, M.H.; Groisman, A.; Kasirer-Friede, A. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab Chip 2008, 8, 1486–1495. [Google Scholar]
- Conant, C.G.; Schwartz, M.A.; Beecher, J.E.; Rudoff, R.C.; Ionescu-Zanetti, C.; Nevill, J.T. Well plate microfluidic system for investigation of dynamic platelet behavior under variable shear loads. Biotechnol. Bioeng 2011, 108, 2978–2987. [Google Scholar]
- Maloney, S.F.; Brass, L.F.; Diamond, S.L. P2Y12 or P2Y1 inhibitors reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Integr. Biol 2010, 2, 183–192. [Google Scholar]
- Colace, T.; Falls, E.; Zheng, X.L.; Diamond, S.L. Analysis of morphology of platelet aggregates formed on collagen under laminar blood flow. Ann. Biomed. Eng 2011, 39, 922–929. [Google Scholar]
- Basmadjian, D. The hemodynamic forces acting on thrombi, from incipient attachment of single cells to maturity and embolization. J. Biomech 1984, 17, 287–298. [Google Scholar]
- Basmadjian, D. The hemodynamic and embolizing forces acting on thrombi—II. The effect of pulsatile blood flow. J. Biomech 1986, 19, 837–845. [Google Scholar]
- Basmadjian, D. Embolization: Critical thrombus height, shear rates, pulsatility. Patency of blood vessels. J. Biomed. Mater. Res 1989, 23, 1315–1326. [Google Scholar]
- Thomas, S.G.; Calaminus, S.D.; Auger, J.M.; Watson, S.P.; Machesky, L.M. Studies on the actin-binding protein HS1 in platelets. BMC Cell Biol 2007, 8. [Google Scholar] [CrossRef]
- Law, D.A.; DeGuzman, F.R.; Heiser, P.; Ministri-Madrid, K.; Killeen, N.; Phillips, D.R. Integrin cytoplasmic tyrosine motif is required for outside-in αIIbβ3 signalling and platelet function. Nature 1999, 401, 808–811. [Google Scholar]
- Suzuki-Inoue, K.; Hughes, C.E.; Inoue, O.; Kaneko, M.; Cuyun-Lira, O.; Takafuta, T.; Watson, S.P.; Ozaki, Y. Involvement of Src kinases and PLCγ2 in clot retraction. Thromb. Res 2007, 120, 251–258. [Google Scholar]
- Katori, N.; Tanaka, K.A.; Szlam, F.; Levy, J.H. The effects of platelet count on clot retraction and tissue plasminogen activator-induced fibrinolysis on thrombelastography. Anesth. Analg 2005, 100, 1781–1785. [Google Scholar]
- Levrat, A.; Gros, A.; Rugeri, L.; Inaba, K.; Floccard, B.; Negrier, C.; David, J.S. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br. J. Anaesth 2008, 100, 792–797. [Google Scholar]
- Rugeri, L.; Levrat, A.; David, J.S.; Delecroix, E.; Floccard, B.; Gros, A.; Allaouchiche, B.; Negrier, C. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J. Thromb. Haemost 2007, 5, 289–295. [Google Scholar]
- Carroll, R.C.; Craft, R.M.; Langdon, R.J.; Clanton, C.R.; Snider, C.C.; Wellons, D.D.; Dakin, P.A.; Lawson, C.M.; Enderson, B.L.; Kurek, S.J. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl. Res 2009, 154, 34–39. [Google Scholar]
- Haenecke, P.; Klouche, M. Thrombelastography today: Practicability and analytical power. Transfus. Med. Hemother 2007, 34, 421–428. [Google Scholar]
- Cohen, I.; de Vries, A. Platelet contractile regulation in an isometric system. Nature 1973, 246, 36–37. [Google Scholar]
- Salganicoff, L.; Loughnane, M.H.; Sevy, R.W.; Russo, M. The platelet strip. I. A low-fibrin contractile model of thrombin-activated platelets. Am. J. Physiol 1985, 249, C279–C287. [Google Scholar]
- Salganicoff, L.; Sevy, R.W. The platelet strip. II. Pharmacomechanical coupling in thrombin-activated human platelets. Am. J. Physiol 1985, 249, C288–C296. [Google Scholar]
- Cohen, I.; Burk, D.L.; White, J.G. The effect of peptides and monoclonal antibodies that bind to platelet glycoprotein IIb-IIIa complex on the development of clot tension. Blood 1989, 73, 1880–1887. [Google Scholar]
- Carr, M.E., Jr; Zekert, S.L. Measurement of platelet-mediated force development during plasma clot formation. Am. J. Med. Sci. 1991, 302, 13–18. [Google Scholar]
- Carr, M.E., Jr. Development of platelet contractile force as a research and clinical measure of platelet function. Cell Biochem. Biophys. 2003, 38, 55–78. [Google Scholar]
- Carr, M.E., Jr; Angchaisuksiri, P.; Carr, S.L.; Martin, E.J. Effect of non-heparin thrombin antagonists on thrombin generation, platelet function, and clot structure in whole blood. Cell Biochem. Biophys. 2003, 39, 89–99. [Google Scholar]
- Carr, M.E., Jr; Hackney, M.H.; Hines, S.J.; Heddinger, S.P.; Carr, S.L.; Martin, E.J. Enhanced platelet force development despite drug-induced inhibition of platelet aggregation in patients with thromboangiitis obliterans—Two case reports. Vasc. Endovascular. Surg. 2002, 36, 473–480. [Google Scholar]
- Greilich, P.E.; Carr, M.E., Jr; Carr, S.L.; Chang, A.S. Reductions in platelet force development by cardiopulmonary bypass are associated with hemorrhage. Anesth. Analg. 1995, 80, 459–465. [Google Scholar]
- Brophy, D.F.; Martin, E.J.; Carr, S.L.; Kirschbaum, B.; Carr, M.E., Jr. The effect of uremia on platelet contractile force, clot elastic modulus and bleeding time in hemodialysis patients. Thromb. Res. 2007, 119, 723–729. [Google Scholar]
- Brophy, D.F.; Martin, E.J.; Best, A.M.; Gehr, T.W.; Carr, M.E. Antifactor Xa activity correlates to thrombin generation time, platelet contractile force and clot elastic modulus following ex vivo enoxaparin exposure in patients with and without renal dysfunction. J. Thromb. Haemost 2004, 2, 1299–1304. [Google Scholar]
- Carr, M.E., Jr; Carr, S.L.; Tildon, T.; Fisher, L.M.; Martin, E.J. Batroxobin-induced clots exhibit delayed and reduced platelet contractile force in some patients with clotting factor deficiencies. J. Thromb. Haemost. 2003, 1, 243–249. [Google Scholar]
- Carr, M.E., Jr; Carr, S.L.; Roa, V.; McCardell, K.A.; Greilich, P.E. Aprotinin counteracts heparin-induced inhibition of platelet contractile force. Thromb. Res. 2002, 108, 161–168. [Google Scholar]
- Krishnaswami, A.; Carr, M.E., Jr; Jesse, R.L.; Kontos, M.C.; Minisi, A.J.; Ornato, J.P.; Vetrovec, G.W.; Martin, E.J. Patients with coronary artery disease who present with chest pain have significantly elevated platelet contractile force and clot elastic modulus. Thromb. Haemost. 2002, 88, 739–744. [Google Scholar]
- Carr, M.E., Jr; Martin, E.J.; Carr, S.L. Delayed, reduced or inhibited thrombin production reduces platelet contractile force and results in weaker clot formation. Blood Coagul. Fibrinolysis 2002, 13, 193–197. [Google Scholar]
- Tan, J.L.; Tien, J.; Pirone, D.M.; Gray, D.S.; Bhadriraju, K.; Chen, C.S. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA 2003, 100, 1484–1489. [Google Scholar]
- Du Roure, O.; Saez, A.; Buguin, A.; Austin, R.H.; Chavrier, P.; Silberzan, P.; Ladoux, B. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA 2005, 102, 2390–2395. [Google Scholar]
- Pirone, D.M.; Liu, W.F.; Ruiz, S.A.; Gao, L.; Raghavan, S.; Lemmon, C.A.; Romer, L.H.; Chen, C.S. An inhibitory role for FAK in regulating proliferation: A link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol 2006, 174, 277–288. [Google Scholar]
- Fu, J.; Wang, Y.K.; Yang, M.T.; Desai, R.A.; Yu, X.; Liu, Z.; Chen, C.S. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 2010, 7, 733–736. [Google Scholar]
- Saez, A.; Buguin, A.; Silberzan, P.; Ladoux, B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys. J 2005, 89, L52–L54. [Google Scholar]
- Sniadecki, N.J.; Lamb, C.M.; Liu, Y.; Chen, C.S.; Reich, D.H. Magnetic microposts for mechanical stimulation of biological cells: Fabrication, characterization, and analysis. Rev. Sci. Instrum 2008, 79. [Google Scholar] [CrossRef]
- Chen, C.S.; Yang, M.T.; Sniadecki, N.J. Geometric considerations of micro- to nanoscale elastomeric post arrays to study cellular traction forces. Adv. Mater 2007, 19, 3119–3123. [Google Scholar]
- Cai, Y.; Biais, N.; Giannone, G.; Tanase, M.; Jiang, G.; Hofman, J.M.; Wiggins, C.H.; Silberzan, P.; Buguin, A.; Ladoux, B.; et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys. J 2006, 91, 3907–3920. [Google Scholar]
- Liu, Z.; Sniadecki, N.J.; Chen, C.S. Mechanical forces in endothelial cells during firm adhesion and early transmigration of human monocytes. Cell Mol. Bioeng 2010, 3, 50–59. [Google Scholar]
- Rabodzey, A.; Alcaide, P.; Luscinskas, F.W.; Ladoux, B. Mechanical forces induced by the transendothelial migration of human neutrophils. Biophys. J 2008, 95, 1428–1438. [Google Scholar]
- Nelson, C.M.; Jean, R.P.; Tan, J.L.; Liu, W.F.; Sniadecki, N.J.; Spector, A.A.; Chen, C.S. Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. USA 2005, 102, 11594–11599. [Google Scholar]
- Ruiz, S.A.; Chen, C.S. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells 2008, 26, 2921–2927. [Google Scholar]
- Kajzar, A.; Cesa, C.M.; Kirchgessner, N.; Hoffmann, B.; Merkel, R. Toward physiological conditions for cell analyses: Forces of heart muscle cells suspended between elastic micropillars. Biophys. J 2008, 94, 1854–1866. [Google Scholar]
- Liu, Z.; Tan, J.L.; Cohen, D.M.; Yang, M.T.; Sniadecki, N.J.; Ruiz, S.A.; Nelson, C.M.; Chen, C.S. Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl. Acad. Sci. USA 2010, 107, 9944–9949. [Google Scholar]
- Maruthamuthu, V.; Sabass, B.; Schwarz, U.S.; Gardel, M.L. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc. Natl. Acad. Sci. USA 2011, 108, 4708–4713. [Google Scholar]
- Ghibaudo, M.; Saez, A.; Trichet, L.; Xayaphoummine, A.; Browaeys, J.; Silberzan, P.; Buguin, A.; Ladoux, B. Traction forces and rigidity sensing regulate cell functions. Soft Matter 2008, 4, 1836–1843. [Google Scholar]
- Sniadecki, N.J.; Anguelouch, A.; Yang, M.T.; Lamb, C.M.; Liu, Z.; Kirschner, S.B.; Liu, Y.; Reich, D.H.; Chen, C.S. Magnetic microposts as an approach to apply forces to living cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14553–14558. [Google Scholar]
- Lemmon, C.A.; Sniadecki, N.J.; Ruiz, S.A.; Tan, J.L.; Romer, L.H.; Chen, C.S. Shear force at the cell-matrix interface: Enhanced analysis for microfabricated post array detectors. Mech. Chem. Biosyst 2005, 2, 1–16. [Google Scholar]
- Kim, K.; Taylor, R.; Sim, J.Y.; Park, S.J.; Norman, J.; Fajardo, G.; Bernstein, D.; Pruitt, B.L. Calibrated micropost arrays for biomechanical characterisation of cardiomyocytes. Micro Nano Lett 2011, 6, 317–322. [Google Scholar]
- Zhao, Y.; Zhang, X. Cellular mechanics study in cardiac myocytes using PDMS pillars array. Sens. Actuators A 2006, 125, 398–404. [Google Scholar]
- Yang, M.T.; Fu, J.; Wang, Y.K.; Desai, R.A.; Chen, C.S. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nat. Protoc 2011, 6, 187–213. [Google Scholar]
- Scheuring, S.; Casuso, I.; Rico, F. Biological AFM: Where we come from—Where we are— Where we may go. J. Mol. Recognit 2011, 24, 406–413. [Google Scholar]
- Radmacher, M. Measuring the elastic properties of biological samples with the AFM. IEEE Eng. Med. Biol. Mag 1997, 16, 47–57. [Google Scholar]
- Rotsch, C.; Radmacher, M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: An atomic force microscopy study. Biophys. J 2000, 78, 520–535. [Google Scholar]
- Mathur, A.B.; Collinsworth, A.M.; Reichert, W.M.; Kraus, W.E.; Truskey, G.A. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J. Biomech 2001, 34, 1545–1553. [Google Scholar]
- Alcaraz, J.; Buscemi, L.; Grabulosa, M.; Trepat, X.; Fabry, B.; Farre, R.; Navajas, D. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys. J 2003, 84, 2071–2079. [Google Scholar]
- Laurent, V.M.; Kasas, S.; Yersin, A.; Schaffer, T.E.; Catsicas, S.; Dietler, G.; Verkhovsky, A.B.; Meister, J.J. Gradient of rigidity in the lamellipodia of migrating cells revealed by atomic force microscopy. Biophys. J 2005, 89, 667–675. [Google Scholar]
- Solon, J.; Levental, I.; Sengupta, K.; Georges, P.C.; Janmey, P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J 2007, 93, 4453–4461. [Google Scholar]
- Maloney, J.M.; Nikova, D.; Lautenschlager, F.; Clarke, E.; Langer, R.; Guck, J.; van Vliet, K.J. Mesenchymal stem cell mechanics from the attached to the suspended state. Biophys. J 2010, 99, 2479–2487. [Google Scholar]
- Cross, S.E.; Jin, Y.S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol 2007, 2, 780–783. [Google Scholar]
- Benoit, M.; Gabriel, D.; Gerisch, G.; Gaub, H.E. Discrete interactions in cell adhesion measured by single-molecule force spectroscopy. Nat. Cell Biol 2000, 2, 313–317. [Google Scholar]
- Helenius, J.; Heisenberg, C.P.; Gaub, H.E.; Muller, D.J. Single-cell force spectroscopy. J. Cell Sci 2008, 121, 1785–1791. [Google Scholar]
- Puech, P.H.; Poole, K.; Knebel, D.; Muller, D.J. A new technical approach to quantify cell-cell adhesion forces by AFM. Ultramicroscopy 2006, 106, 637–644. [Google Scholar]
- Moy, V.T.; Zhang, X.H.; Wojcikiewicz, E. Force spectroscopy of the leukocyte function-associated antigen-1/intercellular adhesion molecule-1 interaction. Biophys. J 2002, 83, 2270–2279. [Google Scholar]
- Rief, M.; Schwaiger, I.; Kardinal, A.; Schleicher, M.; Noegel, A.A. A mechanical unfolding intermediate in an actin-crosslinking protein. Nat. Struct. Mol. Biol 2004, 11, 81–85. [Google Scholar]
- Wojcikiewicz, E.P.; Abdulreda, M.H.; Zhang, X.H.; Moy, V.T. Force spectroscopy of LFA-1 and its ligands, ICAM-1 and ICAM-2. Biomacromolecules 2006, 7, 3188–3195. [Google Scholar]
- Sokurenko, E.V.; Vogel, V.; Thomas, W.E. Catch-bond mechanism of force-enhanced adhesion: Counterintuitive, elusive, but... widespread? Cell Host Microbe 2008, 4, 314–323. [Google Scholar]
- Zhu, C.; Kong, F.; Garcia, A.J.; Mould, A.P.; Humphries, M.J. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol 2009, 185, 1275–1284. [Google Scholar]
- Zhu, C.; Marshall, B.T.; Sarangapani, K.K.; Lou, J.H.; McEver, R.P. Force history dependence of receptor-ligand dissociation. Biophys. J 2005, 88, 1458–1466. [Google Scholar]
- Yago, T.; Lou, J.; Wu, T.; Yang, J.; Miner, J.J.; Coburn, L.; Lopez, J.A.; Cruz, M.A.; Dong, J.F.; McIntire, L.V.; et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J. Clin. Invest 2008, 118, 3195–3207. [Google Scholar]
- Lancaster, C.; Kokoris, A.; Nabavi, M.; Clemmens, J.; Maloney, P.; Capadanno, J.; Gerdes, J.; Battrell, C.F. Rare cancer cell analyzer for whole blood applications: Microcytometer cell counting and sorting subcircuits. Methods 2005, 37, 120–127. [Google Scholar]
- Kokoris, M.; Nabavi, M.; Lancaster, C.; Clemmens, J.; Maloney, P.; Capadanno, J.; Gerdes, J.; Battrell, C.F. Rare cancer cell analyzer for whole blood applications: Automated nucleic acid purification in a microfluidic disposable card. Methods 2005, 37, 114–119. [Google Scholar]
- Prabhakarpandian, B.; Pant, K.; Scott, R.C.; Patillo, C.B.; Irimia, D.; Kiani, M.F.; Sundaram, S. Synthetic microvascular networks for quantitative analysis of particle adhesion. Biomed. Microdevices 2008, 10, 585–595. [Google Scholar]
- Conant, C.G.; Nevill, J.T.; Zhou, Z.; Dong, J.F.; Schwartz, M.A.; Ionescu-Zanetti, C. Using well-plate microfluidic devices to conduct shear-based thrombosis assays. J. Lab. Autom 2011, 16, 148–152. [Google Scholar]
- Nurden, A.T.; Nurden, P. Inherited disorders of platelets: An update. Curr. Opin. Hematol 2006, 13, 157–162. [Google Scholar]
- Nurden, P.; Nurden, A.T. Congenital disorders associated with platelet dysfunctions. Thromb. Haemost 2008, 99, 253–263. [Google Scholar]



© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feghhi, S.; Sniadecki, N.J. Mechanobiology of Platelets: Techniques to Study the Role of Fluid Flow and Platelet Retraction Forces at the Micro- and Nano-Scale. Int. J. Mol. Sci. 2011, 12, 9009-9030. https://doi.org/10.3390/ijms12129009
Feghhi S, Sniadecki NJ. Mechanobiology of Platelets: Techniques to Study the Role of Fluid Flow and Platelet Retraction Forces at the Micro- and Nano-Scale. International Journal of Molecular Sciences. 2011; 12(12):9009-9030. https://doi.org/10.3390/ijms12129009
Chicago/Turabian StyleFeghhi, Shirin, and Nathan J. Sniadecki. 2011. "Mechanobiology of Platelets: Techniques to Study the Role of Fluid Flow and Platelet Retraction Forces at the Micro- and Nano-Scale" International Journal of Molecular Sciences 12, no. 12: 9009-9030. https://doi.org/10.3390/ijms12129009




