Effect of Ligusticum wallichii Aqueous Extract on Oxidative Injury and Immunity Activity in Myocardial Ischemic Reperfusion Rats
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Preparation of Ligusticum wallichii Extract
2.3. Experimental Procedure
2.4. ECG ST-Segment Evaluation
2.5. Samples Preparation
2.6. Biochemical Analysis
2.7. Statistical Analysis
3. Result
3.1. Effects of Pretreatment with Ligusticum wallichii Extract on the ST Segment of ECG in Rats Subjected to Ischemia and Reperfusion
3.2. Effect of Ligusticum wallichii Extract on Serum CK, LDH and AST Activities
3.3. Effect of Ligusticum wallichii Extract on Serum MIP-1α and CRP Levels
3.4. Effect of Ligusticum wallichii Extract on Serum TNF-α, IL-6, IL-8 and Myocardium IL-10 Levels
3.5. Effect of Ligusticum wallichii AExtract on Serum NO Level and Myocardium NOS Activity
3.6. Effect of Ligusticum wallichii Extract on Myocardium MDA and GSH Levels
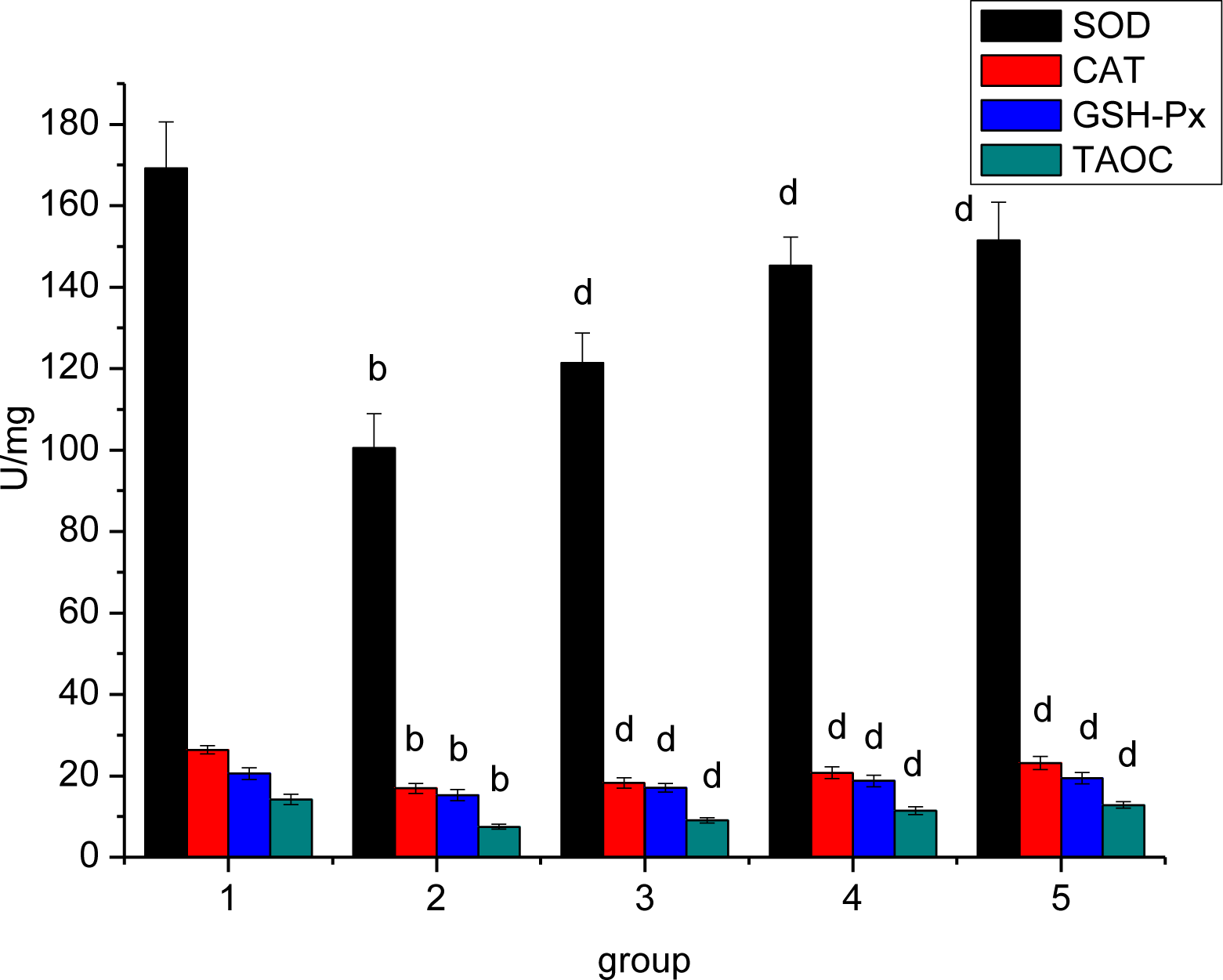
3.7. Effect of Ligusticum wallichii Extract on Myocardium SOD, CAT, GSH-Px and TAOC Activities
3.8. Effect of Ligusticum wallichii Extract on Myocardium Na+-K+-ATPase and Ca2+-Mg2+-ATPase Activities
4. Discussion
5. Conclusion
References
- Li, SZ. Bencao Gangmu (Compendium of Materia Medica); JiLin University Publishing House: ChangChun, China, 2009. [Google Scholar]
- Chiou, GCY; Yan, HY; Lei, HY; Li, BHP; Shen, ZF. Ocular and cardiovascular pharmacology of tetramethylpyrazine isolated from Ligusticum wallichii Franch. Zhong Guo Yao Li Xue Bao 1991, 12, 99–104. [Google Scholar]
- Hwang, KC. The Pharmacology of Chinese Herb; CRC Press: Boca Raton, FL, USA, 1993; p. 84. [Google Scholar]
- Hansen, PR. Myocardial reperfusion injury: experimental evidence and clinical relevance. Eur. Heart J 1995, 16, 734–740. [Google Scholar]
- McCord, JM. Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med 1985, 312, 159–163. [Google Scholar]
- Kloner, RA; Przyklenk, K; Whittaker, P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation 1989, 80, 1115–1126. [Google Scholar]
- Thompson-Gorman, SL; Zweier, JL. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J. Biol. Chem 1990, 265, 6656–6663. [Google Scholar]
- Ruuge, EK; Ledenev, AN; Lakomkin, VL; Konstantinov, AA; Ksenzenko, MY. Free radical metabolites in myocardium during ischemia-reperfusion. Am. J. Physiol 1991, 261, 81–86. [Google Scholar]
- Takemura, G; Onodera, T; Ashraf, M. Quantification of hydroxyl radical and its lack of relevance to myocardial injury during early reperfusion after graded ischemia in rat hearts. Circ. Res 1992, 71, 96–105. [Google Scholar]
- Downey, JM; Omar, B; Ooiwa, H; McCord, J. Superoxide dismutase therapy for myocardial ischemia. Free Radic. Res. Commun 1991, 12, 703–720. [Google Scholar]
- Qiu, Y; Galinanes, M; Ferrari, R; Cargnoni, A; Ezrin, A; Hearse, DJ. PEG-SOD improves postischemic functional recovery and antioxidant status in blood-perfused rabbit hearts. Am. J. Physiol 1992, 263, H1243–H1249. [Google Scholar]
- Haramaki, N; Packer, L; Assadnazari, H; Zimmer, G. Cardiac recovery during post-ischemic reperfusion is improved by combination of vitamin E with dihydrolipoic acid. Biochem. Biophys. Res. Commun 1993, 196, 1101–1107. [Google Scholar]
- Sies, H. Oxidative stress: from basic research to clinical application. Am. J. Med 1991, 91, 31S–38S. [Google Scholar]
- Poltronieri, R; Cevese, A; Sbarbati, A. Protective effect of selenium in cardiac ischemia and reperfusion. Cardioscience 1992, 3, 155–160. [Google Scholar]
- Gross, GJ; Farber, NE; Hardman, HF; Warltier, DC. Beneficial actions of superoxide dismutase and catalase in stunned myocardium of dogs. Am. J. Physiol 1986, 250, 372–377. [Google Scholar]
- Opie, LH. Reperfusion injury and its pharmacologic modification. Circulation 1989, 80, 1049–1062. [Google Scholar]
- Kloner, RA; Przyklenk, K; Whittaker, P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation 1989, 80, 1115–1127. [Google Scholar]
- Kilgore, KS; Lucchesi, BR. Reperfusion injury after myocardial infarction: the role of free radicals and the inflammatory response. Clin. Biochem 1993, 26, 359–370. [Google Scholar]
- Godin, DV; Garnett, ME. Altered antioxidant status in the ischemic/reperfused rabbit myocardium: effects of allopurinol. Can. J. Cardiol 1989, 5, 365–371. [Google Scholar]
- Pyles, LA; Fortney, JE; Kudlak, JJ; Gustafson, RA; Einzig, S. Plasma antioxidant depletion after cardiopulmonary bypass in operations for congenital heart disease. J. Thorac. Cardiovasc. Surg 1995, 110, 165–171. [Google Scholar]
- Ko, KM; Garnett, ME; Godin, DV. Altered antioxidant status in ischemic/reperfused rabbit myocardium: reperfusion time-course study. Can. J. Cardiol 1990, 6, 299–304. [Google Scholar]
- Leichtweis, S; Ji, LL. Glutathione deficiency intensifies ischaemia-reperfusion induced cardiac dysfunction and oxidative stress. Acta Physiol. Scand 2001, 172, 1–10. [Google Scholar]
- Fan, TM; Kranz, DM; Flavell, RA; Roy, EJ. Costimulatory strength influences the differential effects of transforming growth factor beta1 for the generation of CD8+ regulatory T cells. Mol. Immunol 2008, 45, 2937–2950. [Google Scholar]
- Lakoski, SG; Liu, Y; Brosnihan, KB; Herrington, DM. Interleukin-10 concentration and coronary heart disease (CHD) event risk in the estrogen replacement and atherosclerosis (ERA) study. Atherosclerosis 2008, 197, 443–447. [Google Scholar]
- Eefting, D; Schepers, A; de Vries, MR; Pires, NM; Grimbergen, JM; Lagerweij, T; Nagelkerken, LM; Monraats, PS; Jukema, JW; van Bockel, JH; Quax, PH. The effect of interleukin-10 knock-out and overexpression on neointima formation in hypercholesterolemic APOE*3-Leiden mice. Atherosclerosis 2007, 193, 335–342. [Google Scholar]
- Satterthwaite, G; Francis, SE; Suvarna, K; Blakemore, S; Ward, C; Wallace, D; Braddock, M; Crossman, D. Differential gene expression in coronary arteries from patients presenting with ischemic heart disease: further evidence for the inflammatory basis of atherosclerosis. Am. Heart J 2005, 150, 488–499. [Google Scholar]
- de Waal Malefyt, R; Abrams, J; Bennett, B; Figdor, CG; de Vries, JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med 1991, 174, 1209–1220. [Google Scholar]
- Moro, C; Jouan, MG; Rakotovao, A; Toufektsian, MC; Ormezzano, O; Nagy, N; Tosaki, A; de Leiris, J; Boucher, F. Delayed expression of cytokines after reperfused myocardial infarction: possible trigger for cardiac dysfunction and ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol 2007, 293, H3014–H3019. [Google Scholar]
- Cheng, XS; Shimokawa, H; Momii, H; Oyama, J; Fukuyama, N; Egashira, K; Nakazawa, H; Takeshita, A. Role of superoxide anion in the pathogenesis of cytokine-induced myocardial dysfunction in dogs in vivo. Cardiovasc. Res 1999, 42, 651–659. [Google Scholar]
- Hotta, Y; Otsuka-Murakami, H; Fujita, M; Nakagawa, J; Yajima, M; Liu, W; Ishikawa, N; Kawai, N; Masumizu, T; Kohno, M. Protective role of nitric oxide synthase against ischemia-reperfusion injury in guinea pig myocardial mitochondria. Eur. J. Pharmacol 380, 37–48.
- Michel, T; Feron, O. Nitric oxide synthases: which, where, how, and why? J. Clin. Invest 1997, 100, 2146–2152. [Google Scholar]
- Forstermann, U; Pollock, JS; Schmidt, HH; Heller, M; Murad, F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc. Natl. Acad. Sci. USA 1991, 88, 1788–1792. [Google Scholar]
- Hughes, BP. A method for estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathologic sera. Clin. Chim. Acta 1962, 7, 597–604. [Google Scholar]
- Wroblewski, F; Cabaud, PG. Colorimetric measurement of lactic dehydro-genase activity of body fluids. Am. J. Clin. Pathol 1958, 30, 234. [Google Scholar]
- Durak, I; Kavutcu, M; Kaçmaz, M; Avci, A; Horasanli, E; Dikmen, B. Effects of isoflurane on nitric oxide metabolism and oxidant status of rat myocardium. Acta Anaesthesiol. Scand 2001, 45, 119–122. [Google Scholar]
- Buege, A; Aust, S. Microsomal lipid peroxidation. Methods Enzymol 1978, 51, 302–307. [Google Scholar]
- Ellman, GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys 1959, 82, 70–77. [Google Scholar]
- Sun, Y; Oberley, LW; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem 1988, 34, 497–500. [Google Scholar]
- Aebi, HE. Catalase in vitro. Methods Enzymol 1984, 105, 121–126. [Google Scholar]
- Flohe, L; Gunzler, AW. Analysis of glutathione peroxidase. Methods Enzymol 1984, 105, 114–121. [Google Scholar]
- Benzie, IFF; Stain, JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”, the FRAP assay. Anal. Biochem 1996, 239, 70–76. [Google Scholar]
- Tsakiris, S; Deliconstantinos, G. Influence of phosphatidylserine on (Na++K+)-stimulated ATPase and acetylcholonesterase activities of dog brain synaptossomal plasma membranes. Biochem. J 1984, 22, 301–307. [Google Scholar]
- Chan, KM; Delfer, D; Junger, KD. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal. Biochem 1986, 157, 375–380. [Google Scholar]
- Bayer, AJ; Chadha, JS; Farag, RR; Pathy, MSJ. Changing presentation of myocardial infarction with increasing old age. J. Am. Geriatr. Soc 1986, 34, 263–266. [Google Scholar]
- Mathew, S; Menon, PV; Kurup, PA. Effect of administration of vitamin A, ascorbic acid and nicotinamide adenine dinucleotide and flavine adenine nucleotide on severity of myocardial infarction induced by isoproterenol in rats. Indian J. Exp. Biol 1985, 23, 500–504. [Google Scholar]
- Gurevitch, J; Frolkis, I; Yuhas, Y; Lifschitz-Mercer, B; Berger, E; Paz, Y; Matsa, M; Kramer, A; Mohr, R. Anti-tumor necrosis factor-alpha improves myocardial recovery after ischemia and reperfusion. J. Am. Coll. Cardiol 1997, 30, 1554–1561. [Google Scholar]
- Heemann, U; Szabo, A; Hamar, P; Müller, V; Witzke, O; Lutz, J; Philipp, T. Lipopolysaccharide pre-treatment protects from renal ischemia/reperfusion injury. Am. J. Pathol 2000, 156, 287–293. [Google Scholar]
- Deamen, M; van de Ven, M; Heineman, E; Buurman, W. Involvement of endogenous interleukin-10 and tumor necrosis factor-α in renal ischemia-reperfusion injury. Transplantation 1999, 67, 792–800. [Google Scholar]
- Meng, GL; Zhu, HY; Yang, SJ; Wu, F; Zheng, HH; Chen, E; Xu, JL. Attenuating effects of Ganoderma lucidum polysaccharides on myocardial collagen cross-linking relates to advanced glycation end product and antioxidant enzymes in high-fat-diet and streptozotocin-induced diabetic rats. Carbohydr. Polym 2011, 84, 180–185. [Google Scholar]
- Laursen, BE; Stankevicius, E; Pilegaard, H; Mulvany, M; Simonsen, U. Potential protective properties of a stable, slow-releasing nitric oxide donor, GEA 3175, in the lung. Cardiovasc. Drug Rev 2006, 24, 247–260. [Google Scholar]
- Reichenbach, G; Momi, S; Gresele, P. Nitric oxide and its antithrombotic action in the cardiovascular system. Curr. Drug Targets Cardiovasc. Haematol. Disord 2005, 5, 65–74. [Google Scholar]
- Erdmann, E; Philipp, G; Scholz, H. Cardiac glycoside receptor, Na+-K+-ATPase activity and force of contraction in rat heart. Biochem. Pharmacol 1980, 29, 3219–3129. [Google Scholar]
- Jaeschke, H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol 2003, 284, G15–G26. [Google Scholar]
- Fondevila, C; Busuttil, RW; Kupiec-Weglinski, JW. Hepatic ischemia/reperfusion injury—a fresh look. Exp. Mol. Pathol 2003, 74, 86–93. [Google Scholar]






© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zengyong, Q.; Jiangwei, M.; Huajin, L. Effect of Ligusticum wallichii Aqueous Extract on Oxidative Injury and Immunity Activity in Myocardial Ischemic Reperfusion Rats. Int. J. Mol. Sci. 2011, 12, 1991-2006. https://doi.org/10.3390/ijms12031991
Zengyong Q, Jiangwei M, Huajin L. Effect of Ligusticum wallichii Aqueous Extract on Oxidative Injury and Immunity Activity in Myocardial Ischemic Reperfusion Rats. International Journal of Molecular Sciences. 2011; 12(3):1991-2006. https://doi.org/10.3390/ijms12031991
Chicago/Turabian StyleZengyong, Qiao, Ma Jiangwei, and Liu Huajin. 2011. "Effect of Ligusticum wallichii Aqueous Extract on Oxidative Injury and Immunity Activity in Myocardial Ischemic Reperfusion Rats" International Journal of Molecular Sciences 12, no. 3: 1991-2006. https://doi.org/10.3390/ijms12031991
APA StyleZengyong, Q., Jiangwei, M., & Huajin, L. (2011). Effect of Ligusticum wallichii Aqueous Extract on Oxidative Injury and Immunity Activity in Myocardial Ischemic Reperfusion Rats. International Journal of Molecular Sciences, 12(3), 1991-2006. https://doi.org/10.3390/ijms12031991



