Optimization of Lipase Production by Burkholderia sp. Using Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Central Composite Design and Response Surface Analysis
2.2. Verification of Model
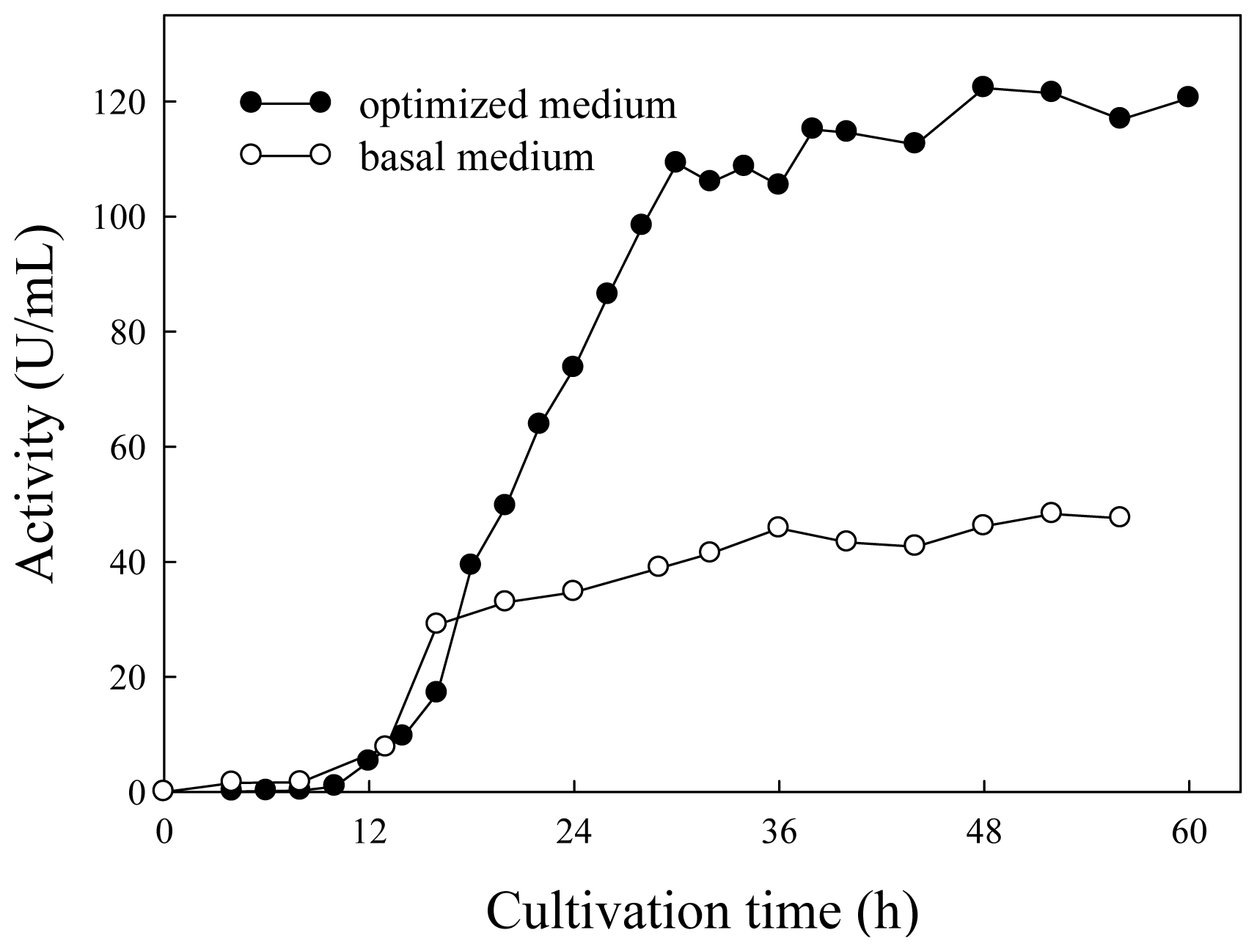
2.3. Lipase Production in Basal and Optimized Media
3. Experimental Section
3.1. Bacterial Strain and Preparation of Inoculum
3.2. Culture Conditions for Lipase Production
3.3. Response Surface Methodology
3.4. Assay for Lipase Activity
4. Conclusions
Acknowledgments
References
- Houde, A.; Kademi, A.; Leblanc, D. Lipases and their industrial applications. Appl. Biochem. Biotechnol 2004, 118, 155–170. [Google Scholar]
- Ribeiro, B.D.; de Castro, A.M.; Coelho, M.A.Z.; Freire, D.M.G. Production and use of lipases in bioenergy: A review from the feedstocks to biodiesel production. Enzyme Res 2011, 2011, 1–16. [Google Scholar]
- Hasan, F.; Shah, A.A.; Javed, S.; Hameed, A. Enzymes used in detergents: Lipases. Afr. J. Biotechnol 2010, 9, 4836–4844. [Google Scholar]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzyme Microb. Technol 2006, 39, 235–251. [Google Scholar]
- Ghosh, P.K.; Saxena, R.K.; Gupta, R.; Yadav, R.P.; Davidson, S. Microbial lipases: Production and applications. Sci. Prog 1996, 79, 119–157. [Google Scholar]
- Soto-Cruz, O.; Saucedo-Castañeda, G.; Pablos-Hach, J.L.; Gutiérrez-Rojas, M.; Favela-Torres, E. Effect of substrate composition on the mycelial growth of Pleurotus ostreatus. An analysis by mixture and response surface methodologies. Process Biochem 1999, 35, 127–133. [Google Scholar]
- Li, Y.; Cui, F.; Liu, Z.; Xu, Y.; Zhao, H. Improvement of xylanase production by Penicillium oxalicum ZH-30 using response surface methodology. Enzym. Microb. Technol 2007, 40, 1381–1388. [Google Scholar]
- Kumari, A.; Mahapatra, P.; Banerjee, R. Statistical optimization of culture conditions by response surface methodology for synthesis of lipase with Enterobacter aerogenes. Braz. Arch. Biol. Technol 2009, 52, 1349–1356. [Google Scholar]
- Yin, X.; You, Q.; Jiang, Z. Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym 2011, 86, 1358–1364. [Google Scholar]
- Gupta, N.; Mehra, G.; Gupta, R. A glycerol-inducible thermostable lipase from Bacillus sp.: Medium optimization by a Plackett–Burman design and by response surface methodology. Can. J. Microbiol 2004, 50, 361–368. [Google Scholar]
- He, Y.-Q.; Tan, T.-W. Use of response surface methodology to optimize culture medium for production of lipase with Candida sp. 99–125. J. Mol. Catal. B Enzym 2006, 43, 9–14. [Google Scholar]
- Ruchi, G.; Anshu, G.; Khare, S.K. Lipase from solvent tolerant Pseudomonas aeruginosa strain: Production optimization by response surface methodology and application. Bioresour. Technol 2008, 99, 4796–4802. [Google Scholar]
- Kumar, R.; Mahajan, S.; Kumar, A.; Singh, D. Identification of variables and value optimization for optimum lipase production by Bacillus pumilus RK31 using statistical methodology. New Biotechnol 2011, 28, 65–71. [Google Scholar]
- Lo, C.-F. Optimal production of lipase from Burkholderia sp. Master’s Thesis, Tatung University, Taipei, Taiwan, 2012. [Google Scholar]
- Rathi, P.; Saxena, R.K.; Gupta, R. A novel alkaline lipase from Burkholderia cepacia for detergent formulation. Process Biochem 2001, 37, 187–192. [Google Scholar]
- Dandavate, V.; Jinjala, J.; Keharia, H.; Madamwar, D. Production, partial purification and characterization of organic solvent tolerant lipase from Burkholderia multivorans V2 and its application for ester synthesis. Bioresour. Technol 2009, 100, 3374–3381. [Google Scholar]
- Cordenons, A.; Gonzalez, R.; Kok, R.; Hellingwerf, K.J.; Nudel, C. Effect of nitrogen sources on the regulation of extracellular lipase production in Acinetobacter calcoaceticus strains. Biotechnol. Lett 1996, 18, 633–638. [Google Scholar]
- Sifour, M.; Zaghloul, T.I.; Saeed, H.M.; Berekaa, M.M.; Abdel-Fattah, Y.R. Enhanced production of lipase by the thermophilic Geobacillus stearothermophilus strain-5 using statistical experimental designs. New Biotechnol 2010, 27, 330–336. [Google Scholar]
- Shabbiri, K.; Adnan, A. Bio-statistically optimized production of lipases by Brevibacterium linens DSM 20158. World Appl. Sci. J 2011, 13, 1059–1066. [Google Scholar]
- Açıkel, Ü.; Erşan, M.; Sağ Açıkel, Y. Optimization of critical medium components using response surface methodology for lipase production by Rhizopus delemar. Food Bioprod. Process. 2010, 88, 31–39. [Google Scholar]
- Gupta, N.; Sahai, V.; Gupta, R. Alkaline lipase from a novel strain Burkholderia multivorans: Statistical medium optimization and production in a bioreactor. Process Biochem 2007, 42, 518–526. [Google Scholar]
- Lima, V.M.G.; Krieger, N.; Sarquis, M.I.M. Effect of nitrogen and carbon sources on lipase production by Penicillium aurantiogriseum. Food Technol. Biotechnol 2003, 41, 105–110. [Google Scholar]
- Turki, S.; Kraeim, I.B.; Weeckers, F.; Thonart, P.; Kallel, H. Isolation of bioactive peptides from tryptone that modulate lipase production in Yarrowia lipolytica. Bioresour. Technol 2009, 100, 2724–2731. [Google Scholar]
- Sidhu, P.; Sharma, R.; Soni, S.; Gupta, J. Production of extracellular alkaline lipase by a new thermophilic Bacillus sp. Folia Microbiol 1998, 43, 51–54. [Google Scholar]
- Iftikhar, T.; Athar, H. Effects of nutrients on the extracellular lipase production by mutant strain of Rhizopus oligosporous TUV-31. Biotechnology 2002, 1, 15–20. [Google Scholar]
- Liu, C.-H.; Lu, W.-B.; Chang, J.-S. Optimizing lipase production of Burkholderia sp. by response surface methodology. Process Biochem 2006, 41, 1940–1944. [Google Scholar]
- Pencreac’h, G.; Baratti, J.C. Activity of Pseudomonas cepacia lipase in organic media is greatly enhanced after immobilization on a polypropylene support. Appl. Microbiol. Biotechnol 1997, 47, 630–635. [Google Scholar]
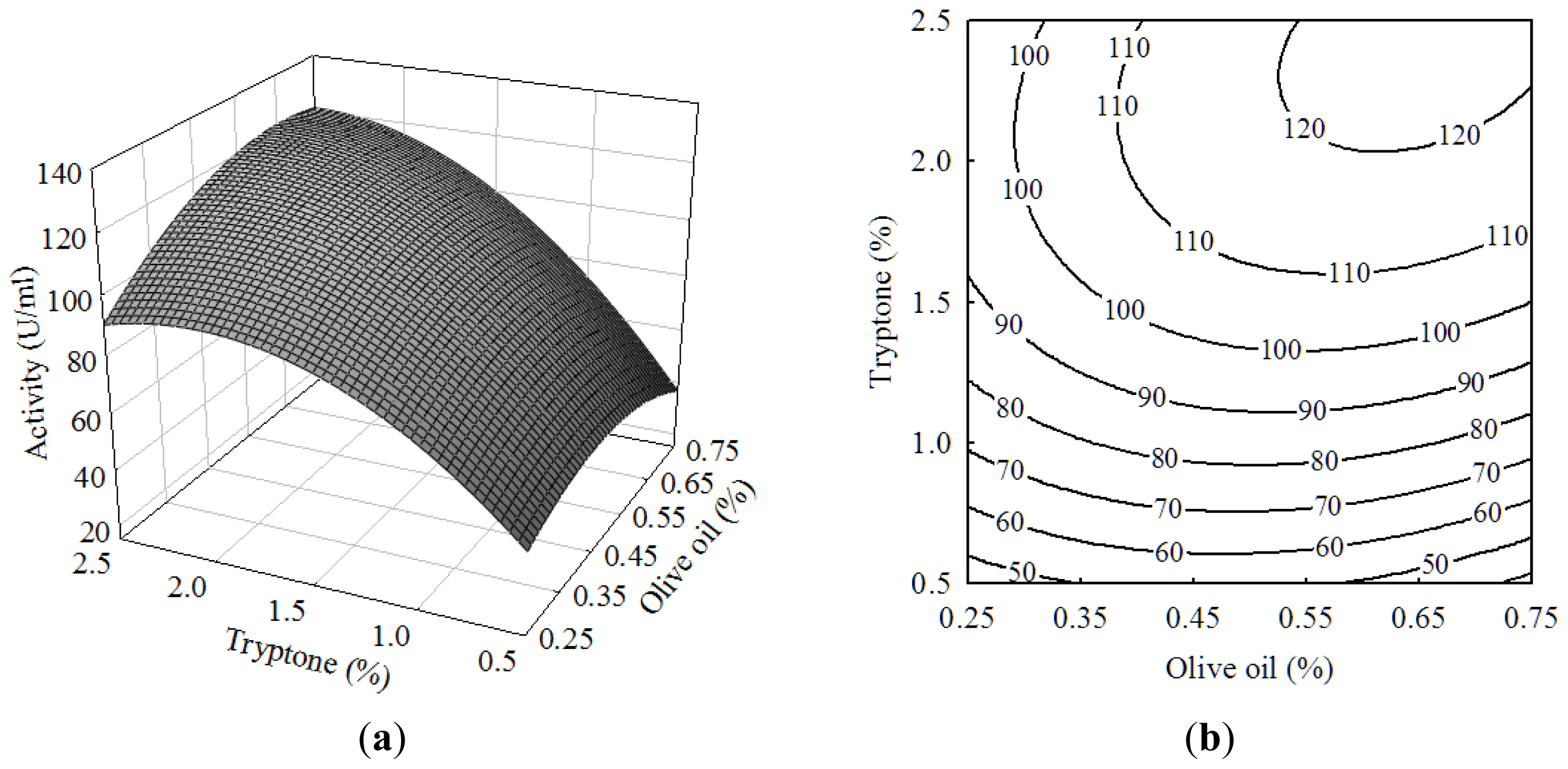

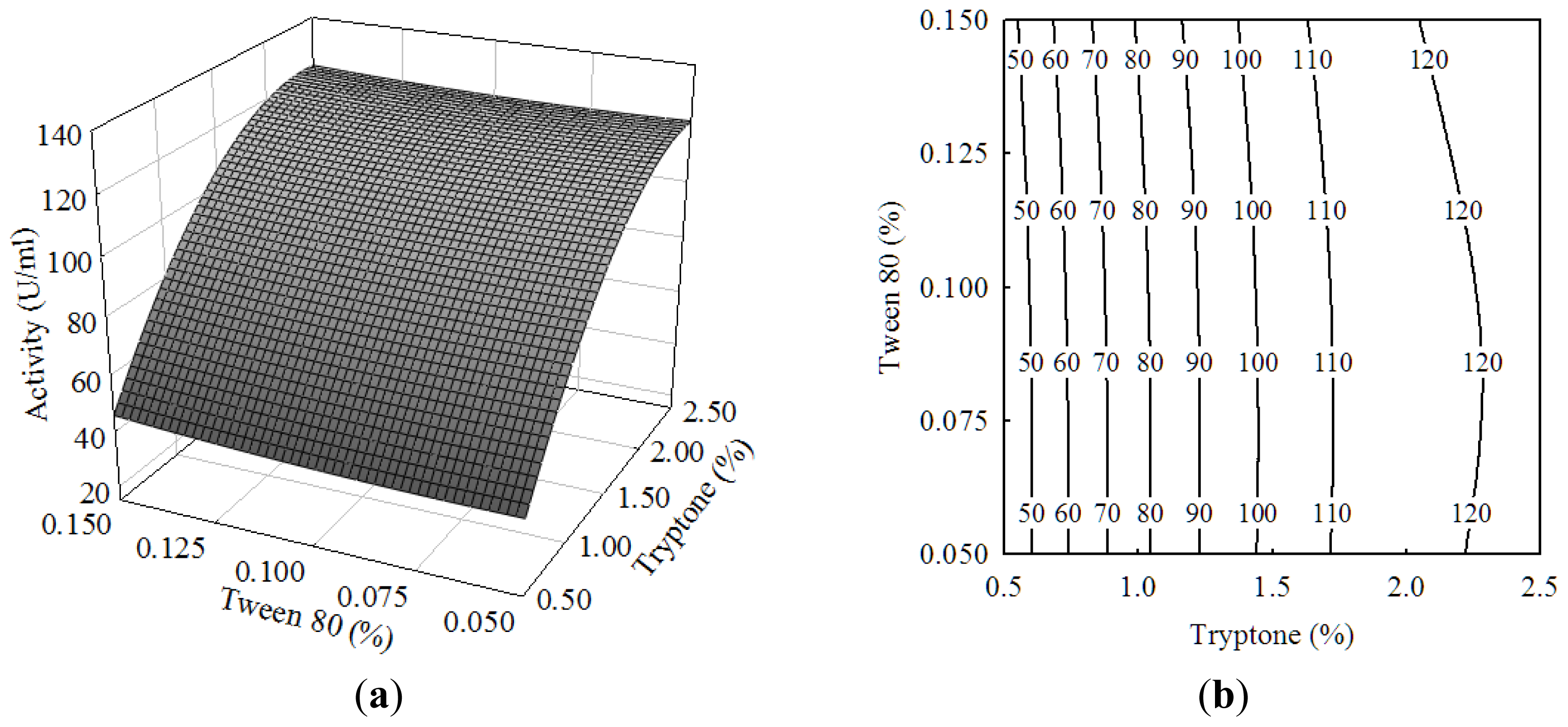
| Run | X1 (olive oil) | X2 (tryptone) | X3 (Tween-80) | Activity (U/mL) | |||
|---|---|---|---|---|---|---|---|
| coded | actual (%) | coded | actual (%) | coded | actual (%) | ||
| 1 | −1 | 0.25 | −1 | 0.5 | −1 | 0.05 | 39.3 |
| 2 | 1 | 0.75 | −1 | 0.5 | −1 | 0.05 | 31.3 |
| 3 | −1 | 0.25 | 1 | 2.5 | −1 | 0.05 | 81.7 |
| 4 | 1 | 0.75 | 1 | 2.5 | −1 | 0.05 | 120.8 |
| 5 | −1 | 0.25 | −1 | 0.5 | 1 | 0.15 | 42.6 |
| 6 | 1 | 0.75 | −1 | 0.5 | 1 | 0.15 | 40.2 |
| 7 | −1 | 0.25 | 1 | 2.5 | 1 | 0.15 | 91.6 |
| 8 | 1 | 0.75 | 1 | 2.5 | 1 | 0.15 | 118.3 |
| 9 | −1 | 0.25 | 0 | 1.5 | 0 | 0.10 | 85.5 |
| 10 | 1 | 0.75 | 0 | 1.5 | 0 | 0.10 | 98.3 |
| 11 | 0 | 0.50 | −1 | 0.5 | 0 | 0.10 | 45.8 |
| 12 | 0 | 0.50 | 1 | 2.5 | 0 | 0.10 | 120.0 |
| 13 | 0 | 0.50 | 0 | 1.5 | −1 | 0.05 | 105.2 |
| 14 | 0 | 0.50 | 0 | 1.5 | 1 | 0.15 | 105.2 |
| 15 | 0 | 0.50 | 0 | 1.5 | 0 | 0.10 | 97.2 |
| 16 | 0 | 0.50 | 0 | 1.5 | 0 | 0.10 | 107.6 |
| 17 | 0 | 0.50 | 0 | 1.5 | 0 | 0.10 | 98.3 |
| 18 | 0 | 0.50 | 0 | 1.5 | 0 | 0.10 | 98.2 |
| 19 | 0 | 0.50 | 0 | 1.5 | 0 | 0.10 | 101.8 |
| 20 | 0 | 0.50 | 0 | 1.5 | 0 | 0.10 | 108.5 |
| Source | Sum of Squares | DF | Mean square | F Value | Probability > F |
|---|---|---|---|---|---|
| Model | 1,6535.33 | 9 | 1837.26 | 82.01 | <0.0001 |
| Residual | 224.03 | 10 | 22.40 | 20.82 | |
| Lack of fit | 99.02 | 5 | 19.80 | 0.79 | 0.5978 |
| Pure error | 125.01 | 5 | 25.0 | ||
| Corrected total | 1,6759.36 | 19 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lo, C.-F.; Yu, C.-Y.; Kuan, I.-C.; Lee, S.-L. Optimization of Lipase Production by Burkholderia sp. Using Response Surface Methodology. Int. J. Mol. Sci. 2012, 13, 14889-14897. https://doi.org/10.3390/ijms131114889
Lo C-F, Yu C-Y, Kuan I-C, Lee S-L. Optimization of Lipase Production by Burkholderia sp. Using Response Surface Methodology. International Journal of Molecular Sciences. 2012; 13(11):14889-14897. https://doi.org/10.3390/ijms131114889
Chicago/Turabian StyleLo, Chia-Feng, Chi-Yang Yu, I-Ching Kuan, and Shiow-Ling Lee. 2012. "Optimization of Lipase Production by Burkholderia sp. Using Response Surface Methodology" International Journal of Molecular Sciences 13, no. 11: 14889-14897. https://doi.org/10.3390/ijms131114889
APA StyleLo, C.-F., Yu, C.-Y., Kuan, I.-C., & Lee, S.-L. (2012). Optimization of Lipase Production by Burkholderia sp. Using Response Surface Methodology. International Journal of Molecular Sciences, 13(11), 14889-14897. https://doi.org/10.3390/ijms131114889




