Dietary Docosahexaenoic Acid (22:6) Incorporates into Cardiolipin at the Expense of Linoleic Acid (18:2): Analysis and Potential Implications
Abstract
:1. Introduction
2. Cardiolipin Response to Dietary Fat Composition
2.1. Dietary Trials Investigating Cardiolipin in Animal Models
2.2. Regulator-Conformer Paradigm
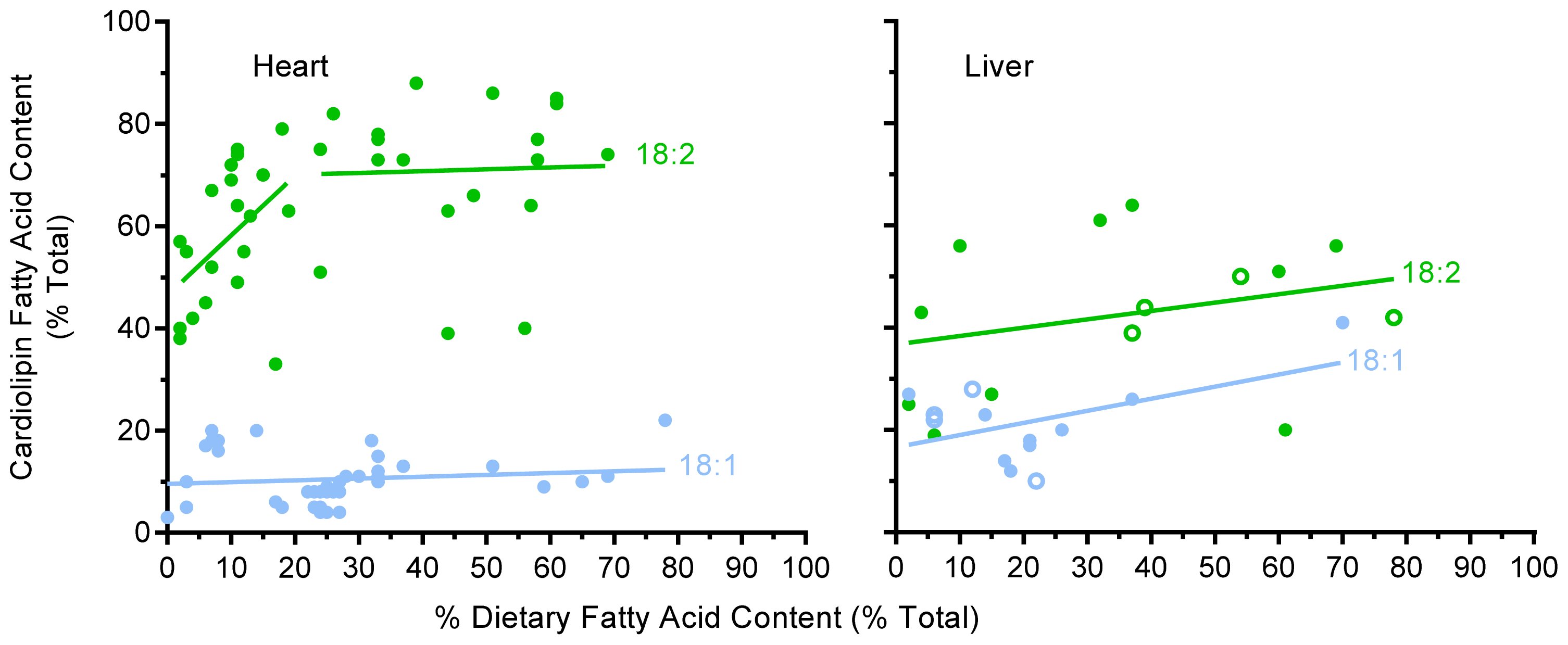
2.3. Unsaturated Fatty Acid Composition of Cardiac Cardiolipin Against Dietary Levels
2.4. Unsaturated Fatty Acid Composition of Hepatic Cardiolipin against Dietary Levels
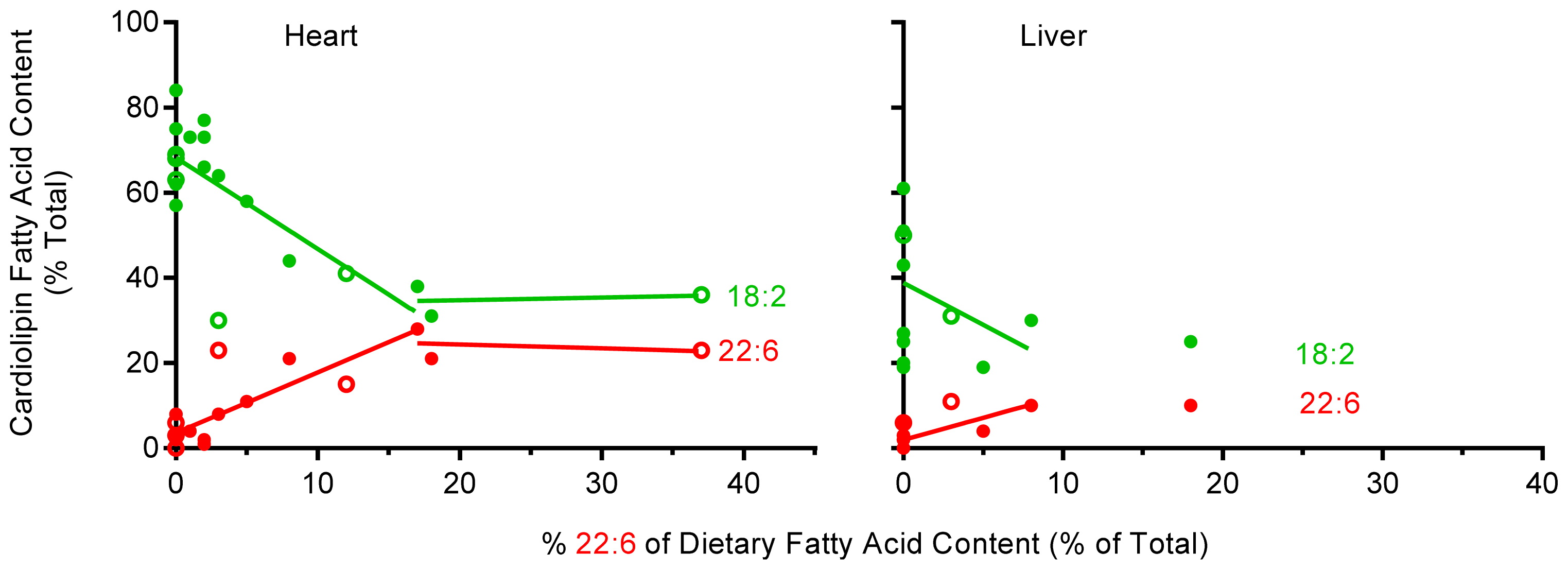
2.5. The Effect of Dietary 22:6 on the Incorporation of 18:2 into Heart and Liver CL
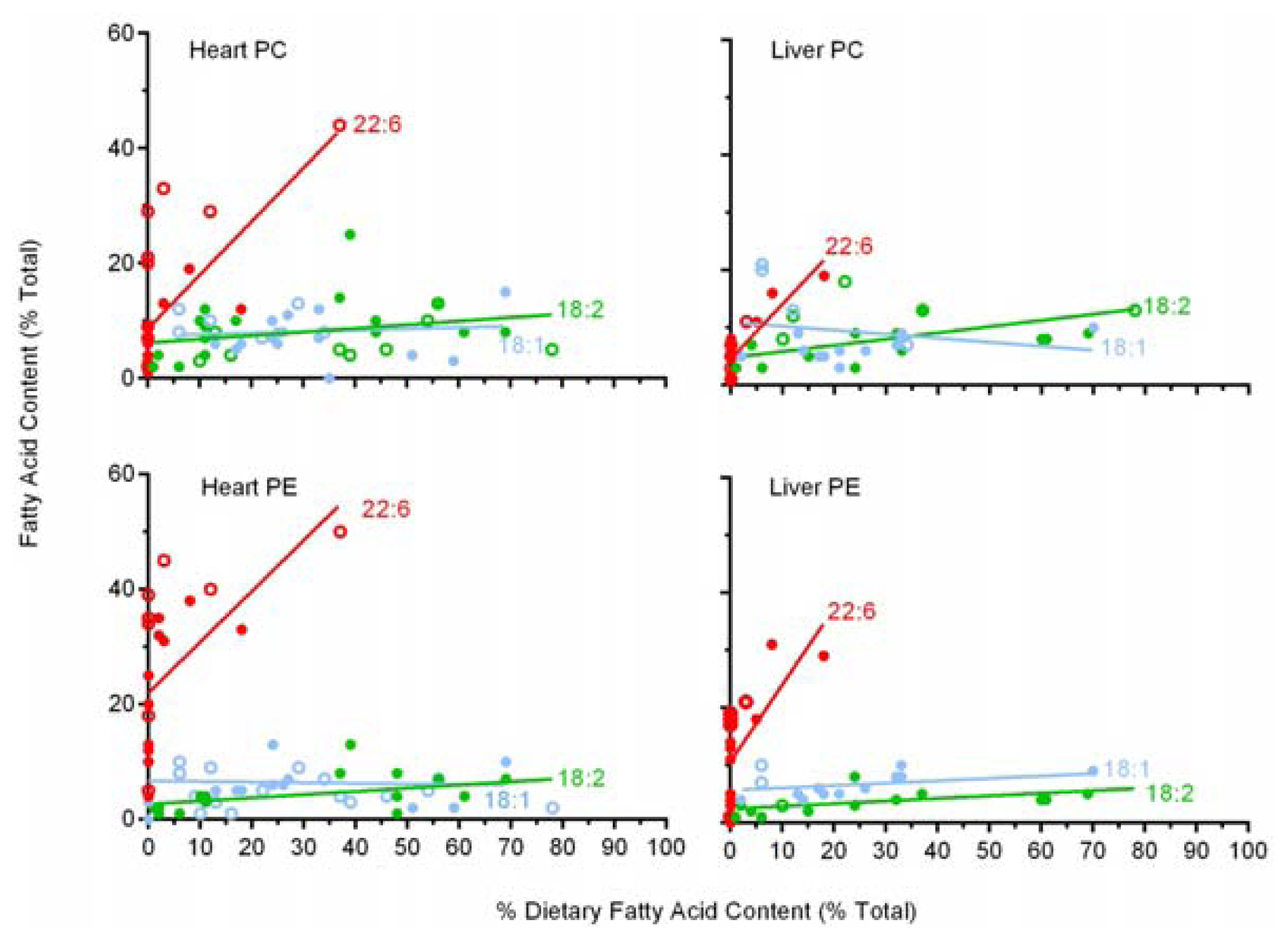
2.6. A Comparison of 22:6 Incorporation into Mitochondrial Phospholipids
3. Consequences of Cardiolipin Composition
4. Conclusions
Acknowledgments
References
- Schlame, M.; Haldar, D. Cardiolipin is synthesized on the matrix side of the inner membrane in rat-liver mitochondria. J. Biol. Chem 1993, 268, 74–79. [Google Scholar]
- Claypool, S.M.; Koehler, C.M. The complexity of cardiolipin in health and disease. Trends Biochem. Sci 2012, 37, 32–41. [Google Scholar]
- Houtkooper, R.H.; Vaz, F.M. Cardiolipin, the heart of mitochondrial metabolism. Cell. Mol. Life Sci 2008, 65, 2493–2506. [Google Scholar]
- Haines, T.H. A new look at cardiolipin. Biochim. Biophys. Acta Biomembr 2009, 1788, 1997–2002. [Google Scholar]
- Koshkin, V.; Greenberg, M.L. Oxidative phosphorylation in cardiolipin-lacking yeast mitochondria. Biochem. J 2000, 347, 687–691. [Google Scholar]
- Claypool, S.M. Cardiolipin, a critical determinant of mitochondrial carrier protein assembly and function. Biochim. Biophys. Acta Biomembr 2009, 1788, 2059–2068. [Google Scholar]
- Kagan, V.E.; Bayir, H.A.; Belikova, N.A.; Kapralov, O.; Tyurina, Y.Y.; Tyurin, V.A.; Jiang, J.F.; Stoyanovsky, D.A.; Wipf, P.; Kochanek, P.M.; et al. Cytochrome c/cardiolipin relations in mitochondria: A kiss of death. Free Radical Biol. Med 2009, 46, 1439–1453. [Google Scholar]
- Nealon, J.R.; Blanksby, S.J.; Mitchell, T.W.; Else, P.L. Systematic differences in membrane acyl composition associated with varying body mass in mammals occur in all phospholipid classes: An analysis of kidney and brain. J. Exp. Biol 2008, 211, 3195–3204. [Google Scholar]
- Norris, S.E.; Mitchell, T.W.; Else, P.L. Phospholipid peroxidation: Lack of effect of fatty acid pairing. Lipids 2012, 47, 451–460. [Google Scholar]
- Schlame, M.; Beyer, K.; Hayerhartl, M.; Klingenberg, M. Molecular-species of cardiolipin in relation to other mitochondrial phospholipids—Is there an acyl specificity of the interaction between cardiolipin and the ADP/ATP carrier? Eur. J. Biochem 1991, 199, 459–466. [Google Scholar]
- Belikova, N.A.; Vladimirov, Y.A.; Osipov, A.N.; Kapralov, A.A.; Tyurin, V.A.; Potapovich, M.V.; Basova, L.V.; Peterson, J.; Kurnikov, I.V.; Kagan, V.E. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry 2006, 45, 4998–5009. [Google Scholar]
- Schlame, M.; Ren, M.D.; Xu, Y.; Greenberg, M.L.; Haller, I. Molecular symmetry in mitochondrial cardiolipins. Chem. Phys. Lipids 2005, 138, 38–49. [Google Scholar]
- Han, X.L.; Yang, K.; Yang, J.Y.; Cheng, H.; Gross, R.W. Shotgun lipidomics of cardiolipin molecular species in lipid extracts of biological samples. J. Lipid Res 2006, 47, 864–879. [Google Scholar]
- Cheng, H.; Mancuso, D.J.; Jiang, X.T.; Guan, S.P.; Yang, J.Y.; Yang, K.; Sun, G.; Gross, R.W.; Han, X.L. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: Temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry 2008, 47, 5869–5880. [Google Scholar]
- Yamaoka, S.; Urade, R.; Kito, M. Mitochondrial-function in rats is affected by modification of membrane phospholipids with dietary sardine oil. J. Nutr 1988, 118, 290–296. [Google Scholar]
- McGee, C.D.; Lieberman, P.; Greenwood, C.E. Dietary fatty acid composition induces comparable changes in cardiolipin fatty acid profile of heart and brain mitochondria. Lipids 1996, 31, 611–616. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Cosgrove, J.; Church, D.; Pryor, W. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids 1987, 22, 299–304. [Google Scholar]
- Tyurin, V.A.; Tyurina, Y.Y.; Kochanek, P.M.; Hamilton, R.; DeKosky, S.T.; Greenberger, J.S.; Bayir, H.; Kagan, V.E. Oxidative lipidomics of programmed cell death. Methods in Enzymol 2008, 442, 375–393. [Google Scholar]
- Tyurina, Y.Y.; Tyurin, V.A.; Kapralova, V.I.; Wasserloos, K.; Mosher, M.; Epperly, M.W.; Greenberger, J.S.; Pitt, B.R.; Kagan, V.E. Oxidative lipidomics of gamma-radiation-induced lung injury: Mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Radiat. Res 2011, 175, 610–621. [Google Scholar]
- Liu, W.; Porter, N.A.; Schneider, C.; Brash, A.R.; Yin, H. Formation of 4-hydroxynonenal from cardiolipin oxidation: Intramolecular peroxyl radical addition and decomposition. Free Radic. Biol. Med 2011, 50, 166–178. [Google Scholar]
- Das, U.N. Essential fatty acids enhance free radical generation and lipid peroxidation to induce apoptosis of tumor cells. Clin. Lipidol 2011, 6, 463–489. [Google Scholar]
- Charnock, J.S.; Abeywardena, M.Y.; McLennan, P.L. Comparative changes in the fatty-acid composition of rat cardiac phospholipids after long-term feeding of sunflower seed oil-supplemented or tuna fish oil-supplemented diets. Ann. Nutr. Metab 1986, 30, 393–406. [Google Scholar]
- Astorg, P.O.; Chevalier, J. Phospholipid fatty acid composition and respiratory properties of heart and liver mitochondria from rats fed with or deprived of linolenic acid. Nutr. Res 1991, 11, 71–77. [Google Scholar]
- Lee, A.G. Lipid-protein interactions in biological membranes: A structural perspective. Biochim. Biophys. Acta Biomembr 2003, 1612, 1–40. [Google Scholar]
- Ikeda, I.; Mitsui, K.; Imaizumi, K. Effect of dietary linoleic, α-linolenic and arachidonic acids on lipid metabolism, tissue fatty acid composition and eicosanoid production in rats. J. Nutr. Sci. Vitaminol 1996, 42, 541–551. [Google Scholar]
- Innis, S.M.; Clandinin, M.T. Dynamic modulation of mitochondrial inner-membrane lipids in rat-heart by dietary-fat. Biochem. J 1981, 193, 155–167. [Google Scholar]
- Javouhey, A.; Rocquelin, G.; Rochette, L.; Juaneda, P. Comparative effects of equivalent intakes of 18-3 (n − 3) and of marine (n − 3) fatty-acids on rat cardiac phospholipid contents and fatty-acid compositions. Nutr. Res 1990, 10, 291–301. [Google Scholar]
- Kramer, J.K.G. Comparative studies on composition of cardiac phospholipids in rats fed different vegetable-oils. Lipids 1980, 15, 651–660. [Google Scholar]
- Novak, F.; Tvrzicka, E.; Hamplova, B.; Kolar, F.; Novakova, O. Postnatal development of phospholipids and their fatty acid profile in rat heart. Mol. Cell. Biochem 2006, 293, 23–33. [Google Scholar]
- Power, G.W.; Yaqoob, P.; Harvey, D.J.; Newsholme, E.A.; Calder, P.C. The effect of dietary-lipid manipulation on hepatic mitochondrial phospholipid fatty-acid composition and carnitine palmitoyltransferase-I activity. Biochem. Mol. Biol. Int 1994, 34, 671–684. [Google Scholar]
- Swanson, J.E.; Kinsella, J.E. Dietary normal-3 polyunsaturated fatty-acids —Modification of rat cardiac lipids and fatty-acid composition. J. Nutr 1986, 116, 514–523. [Google Scholar]
- Taniguchi, H.; Suzuki, K.; Takita, T.; Chung, S.Y.; Hayakawa, T.; Nakamura, K.; Innami, S. Comparative effects of eicosapentaenoic acid and docosahexaenoic acid on n-6 and n-3 fatty-acid profiles of phospholipid classes in several tissues of rats fed a hypertriglyceridemic diet. J. Clin. Biochem. Nutr 1993, 14, 151–162. [Google Scholar]
- Yamaoka, S.; Urade, R.; Kito, M. Cardiolipin molecular-species in rat-heart mitochondria are sensitive to essential fatty-acid deficient dietary lipids. J. Nutr 1990, 120, 415–421. [Google Scholar]
- Hoy, C.E.; Holmer, G. Influence of dietary linoleic-acid and trans-fatty-acids on the fatty-acid profile of cardiolipins in rats. Lipids 1990, 25, 455–459. [Google Scholar]
- Charnock, J.S.; Abeywardena, M.Y.; McMurchie, E.J.; Russell, G.R. The composition of cardiac phospholipids in rats fed different lipid supplements. Lipids 1984, 19, 206–213. [Google Scholar]
- Charnock, J.S.; Abeywardena, M.Y.; Tan, D.; McLennan, P.L. Omega-3 and omega-6 PUFAs have different effects on the phospholipid fatty-acid composition of rat myocardial muscle when added to a saturated fatty-acid dietary-supplement. Nutr. Res 1991, 11, 1013–1024. [Google Scholar]
- Robblee, N.M.; Clandinin, M.T. Effect of dietary-fat level and poly-unsaturated fatty-acid content on the phospholipid-composition of rat cardiac mitochondrial-membranes and mitochondrial ATPase activity. J. Nutr 1984, 114, 263–269. [Google Scholar]
- Croset, M.; Kinsella, J.E. Changes in phospholipid fatty-acid composition of mouse cardiac organelles after feeding graded amounts of docosahexaenoate in presence of high-levels of linoleate—Effect on cardiac ATPase activities. Ann. Nutr. Metab 1989, 33, 125–142. [Google Scholar]
- Berger, A.; German, J.B. Phospholipid fatty-acid composition of various mouse-tissues after feeding α-linolenate (18:3n − 3) or eicosatrienoate (20:3n − 3). Lipids 1990, 25, 473–480. [Google Scholar]
- Watkins, S.M.; Lin, T.Y.; Davis, R.M.; Ching, J.R.; DePeters, E.J.; Halpern, G.M.; Walzem, R.L.; German, J.B. Unique phospholipid metabolism in mouse heart in response to dietary docosahexaenoic or α-linolenic acids. Lipids 2001, 36, 247–254. [Google Scholar]
- Berger, A.; Gershwin, M.E.; German, J.B. Effects of various dietary fats on cardiolipin acyl composition during ontogent of mice. Lipids 1992, 27, 605–612. [Google Scholar]
- Hussein, N.; Fedorova, I.; Moriguchi, T.; Hamazaki, K.; Kim, H.-Y.; Hoshiba, J.; Salem, N. Artificial rearing of infant mice leads to n-3 fatty acid deficiency in cardiac, neural and peripheral tissues. Lipids 2009, 44, 685–702. [Google Scholar]
- Burr, G.; Burr, M. On the nature and role of the fatty acids essential in nutrition. J. Biol. Chem 1930, 86, 587–621. [Google Scholar]
- Lee, H.J.; Mayette, J.; Rapoport, S.I.; Bazinet, R.P. Selective remodeling of cardiolipin fatty acids in the aged rat heart. Lipids Health Dis. 2006, 5. [Google Scholar] [CrossRef]
- Abbott, S.K.; Else, P.L.; Hulbert, A.J. Membrane fatty acid composition of rat skeletal muscle is most responsive to the balance of dietary n-3 and n-6 PUFA. Br. J. Nutr 2010, 103, 522–529. [Google Scholar]
- Hulbert, A.J.; Turner, N.; Storlien, L.H.; Else, P.L. Dietary fats and membrane function: Implications for metabolism and disease. Biol. Rev 2005, 80, 155–169. [Google Scholar]
- Abbott, S.K.; Else, P.L.; Atkins, T.A.; Hulbert, A.J. Fatty acid composition of membrane bilayers: Importance of diet polyunsaturated fat balance. Biochim. Biophys. Acta Biomembr 2012, 1818, 1309–1317. [Google Scholar]
- Ailhaud, G.; Massiera, F.; Weill, P.; Legrand, P.; Alessandri, J.M.; Guesnet, P. Temporal changes in dietary fats: Role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog. Lipid Res 2006, 45, 203–236. [Google Scholar]
- Owen, A.J.; Peter-Przyborowska, B.A.; Hoy, A.J.; McLennan, P.L. Dietary fish oil dose- and time-response effects on cardiac phospholipid fatty acid composition. Lipids 2004, 39, 955–961. [Google Scholar]
- Oberley, T.D.; Oberley, L.W. Antioxidant enzyme levels in cancer. Histol. Histopath 1997, 12, 525–535. [Google Scholar]
- Power, G.W.; Newsholme, E.A. Dietary fatty acids influence the activity and metabolic control of mitochondrial carnitine palmitoyltransferase I in rat heart and skeletal muscle. J. Nutr 1997, 127, 2142–2150. [Google Scholar]
- Gao, F.; Kiesewetter, D.; Chang, L.; Ma, K.; Bell, J.M.; Rapoport, S.I.; Igarashi, M. Whole-body synthesis-secretion rates of long-chain n-3 Pufas from circulating unesterified α-linolenic acid in unanesthetized rats. J. Lipid Res 2009, 50, 749–758. [Google Scholar]
- Daum, G.; Vance, J.E. Import of lipids into mitochondria. Prog. Lipid Res 1997, 36, 103–130. [Google Scholar]
- Kiebish, M.A.; Bell, R.; Yang, K.; Phan, T.; Zhao, Z.D.; Ames, W.; Seyfried, T.N.; Gross, R.W.; Chuang, J.H.; Han, X.L. Dynamic simulation of cardiolipin remodeling: Greasing the wheels for an interpretative approach to lipidomics. J. Lipid Res 2010, 51, 2153–2170. [Google Scholar]
- Hermansson, M.; Hokynar, K.; Somerharju, P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid Res 2011, 50, 240–257. [Google Scholar]
- Khairallah, R.J.; Kim, J.; O’Shea, K.M.; O’Connell, K.A.; Brown, B.H.; Galvao, T.; Daneault, C.; Des Rosiers, C.; Polster, B.M.; Hoppel, C.L.; et al. Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids. PLoS One 2012, 7, e3440. [Google Scholar]
- Aoun, M.; Feillet-Coudray, C.; Fouret, G.; Chabi, B.; Crouzier, D.; Ferreri, C.; Chatgilialoglu, C.; Wrutniak-Cabello, C.; Cristol, J.P.; Carbonneau, M.A.; et al. Rat liver mitochondrial membrane characteristics and mitochondrial functions are more profoundly altered by dietary lipid quantity than by dietary lipid quality: Effect of different nutritional lipid patterns. Br. J. Nutr 2012, 107, 647–659. [Google Scholar]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev 2011, 111, 5944–5972. [Google Scholar]
- Gueraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res 2010, 44, 1098–1124. [Google Scholar]
- Negre-Salvayre, A.; Auge, N.; Ayala, V.; Basaga, H.; Boada, J.; Brenke, R.; Chapple, S.; Cohen, G.; Feher, J.; Grune, T.; et al. Pathological aspects of lipid peroxidation. Free Radic. Res 2010, 44, 1125–1171. [Google Scholar]
- Colas, S.; Maheo, K.; Denis, F.; Goupille, C.; Hoinard, C.; Champeroux, P.; Tranquart, F.; Bougnoux, P. Sensitization by dietary docosahexaenoic acid of rat mammary carcinoma to anthracycline: A role for tumor vascularization. Clin. Cancer Res 2006, 12, 5879–5886. [Google Scholar]
- Wiswedel, I.; Gardemann, A.; Storch, A.; Peter, D.; Schild, L. Degradation of phospholipids by oxidative stress-exceptional significance of cardiolipin. Free Radic. Res 2010, 44, 135–145. [Google Scholar]
- Li, J.; Romestaing, C.; Han, X.L.; Li, Y.A.; Hao, X.B.; Wu, Y.Y.; Sun, C.; Liu, X.L.; Jefferson, L.S.; Xiong, J.W.; et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab 2010, 12, 154–165. [Google Scholar]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.F.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V.; et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol 2005, 1, 223–232. [Google Scholar]
- Balakrishnan, G.; Hu, Y.; Oyerinde, O.F.; Su, J.; Groves, J.T.; Spiro, T.G. A conformational switch to β-sheet structure in cytochrome c leads to heme exposure. Implications for cardiolipin peroxidation and apoptosis. J. Am. Chem. Soc 2007, 129, 504–505. [Google Scholar]
- Korytowski, W.; Basova, L.V.; Pilat, A.; Kernstock, R.M.; Girotti, A.W. Permeabilization of the mitochondrial outer membrane by Bax/Truncated Bid (Tbid) proteins as sensitized by cardiolipin hydroperoxide translocation mechanistic implications for the intrinsic pathway of oxidative apoptosis. J. Biol. Chem 2011, 286, 26334–26343. [Google Scholar]
- Watkins, S.M.; Carter, L.C.; German, J.B. Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J. Lipid Res 1998, 39, 1583–1588. [Google Scholar]
- Long, E.K.; Picklo, M.J. Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: make some room HNE. Free Radical Biol. Med 2010, 49, 1–8. [Google Scholar]
- Peoples, G.E.; McLennan, P.L. Dietary fish oil reduces skeletal muscle oxygen consumption, provides fatigue resistance and improves contractile recovery in the rat in vivo hindlimb. Br. J. Nutr 2010, 104, 1771–1779. [Google Scholar]
- Else, P.L.; Turner, N.; Hulbert, A.J. The evolution of endothermy: Role for membranes and molecular activity. Physiol. Biochem. Zool 2004, 77, 950–958. [Google Scholar]
- Das, U.N.; Madhavi, N. Effect of polyunsaturated fatty acids on drug-sensitive and resistant tumor cells in vitro. Lipids Health Dis 2011, 10, 159. [Google Scholar]
- Patterson, R.E.; Flatt, S.W.; Newman, V.A.; Natarajan, L.; Rock, C.L.; Thomson, C.A.; Caan, B.J.; Parker, B.A.; Pierce, J.P. Marine fatty acid intake is associated with breast cancer prognosis. J. Nutr 2011, 141, 201–206. [Google Scholar]
- Serini, S.; Fasano, E.; Piccioni, E.; Cittadini, A.R.M.; Calviello, G. Differential anti-cancer effects of purified EPA and DHA and possible mechanisms involved. Curr. Med. Chem 2011, 18, 4065–4075. [Google Scholar]
- Gleissman, H.; Johnsen, J.I.; Kogner, P. Omega-3 fatty acids in cancer, the protectors of good and the killers of evil? Exp. Cell Res 2010, 316, 1365–1373. [Google Scholar]
- Hajjaji, N.; Schubnel, V.; Bougnoux, P. Determinants of DHA incorporation into tumor tissue during dietary DHA supplementation. Lipids 2011, 46, 1063–1069. [Google Scholar]
- Kiebish, M.A.; Han, X.L.; Cheng, H.; Chuang, J.H.; Seyfried, T.N. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: Lipidomic evidence supporting the warburg theory of cancer. J. Lipid Res 2008, 49, 2545–2556. [Google Scholar]
- Kiebish, M.A.; Han, X.L.; Cheng, H.; Seyfried, T.N. In vitro growth environment produces lipidomic and electron transport chain abnormalities in mitochondria from non-tumorigenic astrocytes and brain tumours. ASN Neuro 2009, 1, e00011. [Google Scholar]
- Schild, L.; Lendeckel, U.; Gardemann, A.; Wiswedel, I.; Schmidt, C.A.; Wolke, C.; Walther, R.; Grabarczyk, P.; Busemann, C. Composition of molecular cardiolipin species correlates with proliferation of lymphocytes. Exp. Biol. Med 2012, 237, 372–379. [Google Scholar]
- Warburg, E. On the origins of cancer cells. Science 1956, 123, 309–314. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar]
| Reference | Source of fat | Measurement method | Dietary fat (w/w) | Feeding period (weeks) |
|---|---|---|---|---|
| Rats | ||||
| Yamaoka et al., 1988 [15] | Corn oil, sardine oil | TLC, GC | 20% | 4 |
| McGee et al., 1996 [16] | Not stated | TLC, GC | 20% | 4 |
| Charnock et al., 1986 [23] | Sunflower oil, tuna, vegetable oil | TLC, GC | 4% or 16% | 60 |
| Astorg et al., 1991 [24] | Sunflower seed oil, linseed oil | HPLC/GC 10% | 20 | |
| Ikeda et al., 1996 [26] | Safflower oil, perilla oil, palm oil, ethyl 20:4 | TLC/[15]GC | 10% | 3 |
| Innis and Clandinin, 1981 [27] | Soya-bean, rapeseed | TLC, GC | 20% | 5 |
| Jahouvey et al., 1990 [28] | Palm oil, olive oil, sunflower oil, linseed oil, menhaden oil | HPLC, GC | 15% | 4 |
| Kramer, 1980 [29] | Corn oil, zephyr oil | TLC, GC | 20% | 16 |
| Novak et al., 2006 [30] | Standard chow diet | TLC, GC | 3.5% | 9 |
| Power et al., 1994 [31] | Coconut oil, olive oil, safflower oil, menhaden oil | TLC, GC | 2% or 20% | 10 |
| Taniguchi et al., 1993 [33] | 20:5 and 22:6 methylesters | TLC, GC | 15% | 2 |
| Yamaoka et al., 1990 [34] | Corn oil, sardine oil | TLC, HPLC, GC | 20% | 2 |
| Hoy and Holmer, 1990 [35] | Marine oil, olive oil, sunflower seed oil | TLC, GC | 20% | 10 |
| Charnock et al., 1984 [36] | Sunflower seed oil, sheep kidney fat | TLC, GC | 4%, 12% | 20 |
| Charnock et al., 1991 [37] | Sunflower, fish oil | TLC, GC | 16% | 44 |
| Robblee and Clandinin, 1984 [38] | Beef tallow, soybean oil | TLC, GC | 7%, 7.5%, 21% or 23% | 2 |
| Lee et al., 2006 [45] | Soybean oil, fish meal, soybean meal, alfalfa meal, corn meal | TLC, GC | 4% | 16 |
| Mice | ||||
| Croset and Kinsella, 1989 [39] | Ethyl esters of 18:2, 22:6 | HPTLC, GC | 10% | 2 |
| Berger and German, 1990 [40] | Safflower oil, free fatty acids of 18:2, 20:5 | HPTLC, GC | 2% | 2 |
| Watkins et al., 2001 [41] | Crocodile oil, soybean oil | TLC, GC | 7% | 13 |
| Hussein et al., 2009 [43] | Coconut oil, safflower oil, flaxseed oil | HPLC, MS | 10% | 17 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cortie, C.H.; Else, P.L. Dietary Docosahexaenoic Acid (22:6) Incorporates into Cardiolipin at the Expense of Linoleic Acid (18:2): Analysis and Potential Implications. Int. J. Mol. Sci. 2012, 13, 15447-15463. https://doi.org/10.3390/ijms131115447
Cortie CH, Else PL. Dietary Docosahexaenoic Acid (22:6) Incorporates into Cardiolipin at the Expense of Linoleic Acid (18:2): Analysis and Potential Implications. International Journal of Molecular Sciences. 2012; 13(11):15447-15463. https://doi.org/10.3390/ijms131115447
Chicago/Turabian StyleCortie, Colin H., and Paul L. Else. 2012. "Dietary Docosahexaenoic Acid (22:6) Incorporates into Cardiolipin at the Expense of Linoleic Acid (18:2): Analysis and Potential Implications" International Journal of Molecular Sciences 13, no. 11: 15447-15463. https://doi.org/10.3390/ijms131115447




