Associations between Endogenous Dimethylarginines and Renal Function in Healthy Children and Adolescents
Abstract
:1. Introduction
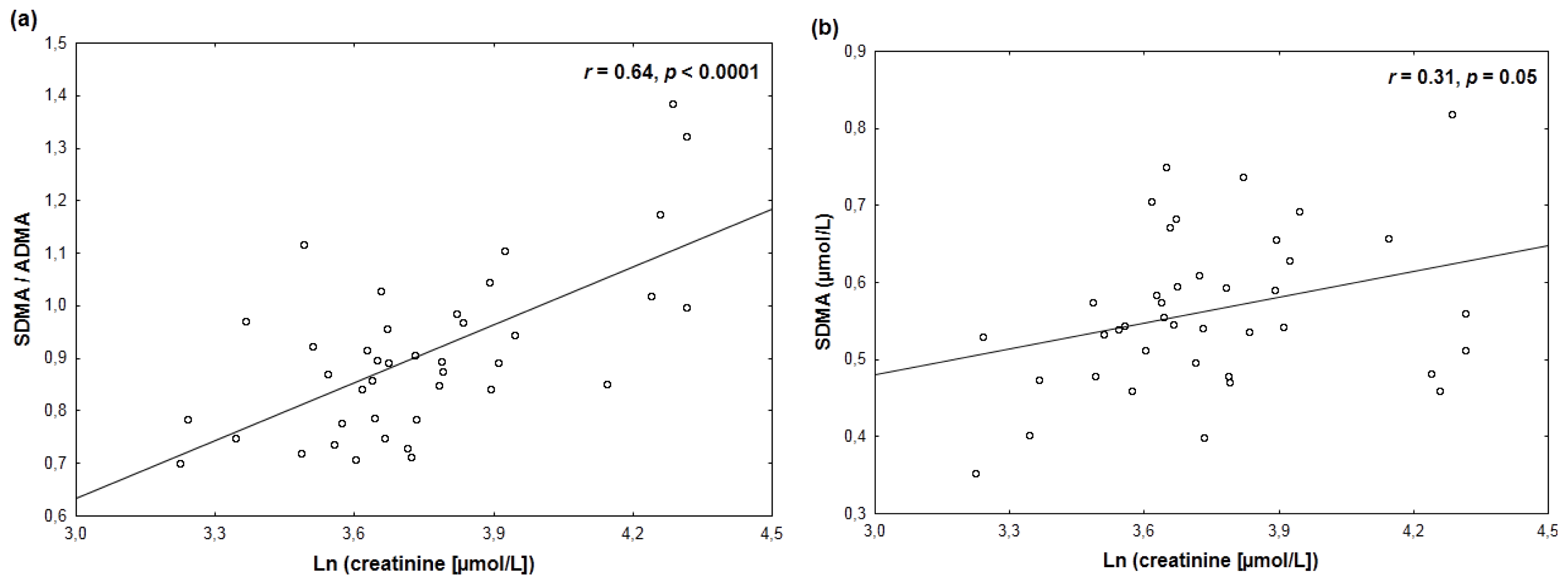
2. Results
3. Discussion
3.1. Comparison with Other Studies Relating Renal Function to Endogenous Dimethylarginines in Children
3.2. Proposed Mechanisms of the Close Relationship between Renal Function Indices and the SDMA to ADMA Ratio
3.3. Study Limitations
4. Experimental Section
4.1. Study Subjects
4.2. Study Protocol
4.3. Statistical Analysis
5. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Bode-Böger, S.M.; Scalera, F.; Kielstein, J.T.; Martens-Lobenhoffer, J.; Breithardt, G.; Fobker, M.; Reinecke, H. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J. Am. Soc. Nephrol 2006, 17, 1128–1134. [Google Scholar]
- Kielstein, J.T.; Fliser, D.; Veldink, H. Asymmetric dimethylarginine and symmetric dimethylarginine: axis of evil or useful alliance? Semin. Dial 2009, 22, 346–350. [Google Scholar]
- Marescau, B.; Nagels, G.; Possemiers, I.; De Broe, M.E.; Becaus, I.; Billiouw, J.M.; Lornoy, W.; De Deyn, P.P. Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism 1997, 46, 1024–1031. [Google Scholar]
- Fliser, D.; Kronenberg, F.; Kielstein, J.T.; Morath, C.; Bode-Böger, S.M.; Haller, H.; Ritz, E. Asymmetric dimethylarginine and progression of chronic kidney disease: The mild to moderate kidney disease study. J. Am. Soc. Nephrol 2005, 16, 2456–2461. [Google Scholar]
- Kielstein, J.T.; Salpeter, S.R.; Bode-Böger, S.M.; Cooke, J.P.; Fliser, D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—A meta-analysis. Nephrol. Dial. Transplant 2006, 21, 2446–2451. [Google Scholar]
- Goonasekera, C.D.; Rees, D.D.; Woolard, P.; Frend, A.; Shah, V.; Dillon, M.J. Nitric oxide synthase inhibitors and hypertension in children and adolescents. J. Hypertens 1997, 15, 901–909. [Google Scholar]
- Brooks, E.R.; Langman, C.B.; Wang, S.; Price, H.E.; Hodges, A.L.; Darling, L.; Yang, A.Z.; Smith, F.A. Methylated arginine derivatives in children and adolescents with chronic kidney disease. Pediatr. Nephrol 2009, 24, 129–134. [Google Scholar]
- Wasilewska, A.; Taranta-Janusz, K.; Zoch-Zwierz, W.; Michaluk-Skutnik, J. Is plasma symmetric dimethylarginine a suitable marker of renal function in children and adolescents? Scand. J. Urol. Nephrol 2012, 46, 58–64. [Google Scholar]
- Heilman, K.; Zilmer, M.; Zilmer, K.; Kool, P.; Tillmann, V. Elevated plasma adiponectin and decreased plasma homocysteine and asymmetric dimethylarginine in children with type 1 diabetes. Scand. J. Clin. Lab. Invest 2009, 69, 85–91. [Google Scholar]
- Marcovecchio, M.L.; Dalton, R.N.; Turner, C.; Prevost, A.T.; Widmer, B.; Amin, R.; Dunger, D.B. Symmetric dimethylarginine, an endogenous marker of glomerular filtration rate, and the risk for microalbuminuria in young people with type 1 diabetes. Arch. Dis. Child 2010, 95, 119–124. [Google Scholar]
- Marcovecchio, M.L.; Widmer, B.; Turner, C.; Dunger, D.B.; Dalton, R.N. Asymmetric dimethylarginine in young people with Type 1 diabetes: A paradoxical association with HbA(1c). Diabet. Med 2011, 28, 685–691. [Google Scholar]
- Lücke, T.; Kanzelmeyer, N.; Kemper, M.J.; Tsikas, D.; Das, A.M. Developmental changes in the L-arginine/nitric oxide pathway from infancy to adulthood: Plasma asymmetric dimethylarginine levels decrease with age. Clin. Chem. Lab. Med 2007, 45, 1525–1530. [Google Scholar]
- Huemer, M.; Simma, B.; Mayr, D.; Mühl, A.; Rami, B.; Schober, E.; Ulmer, H.; Zanier, U.; Bodamer, O.A. Low levels of asymmetric dimethylarginine in children with diabetes mellitus type I compared with healthy children. J. Pediatr 2011, 158, 602–606. [Google Scholar]
- Aldámiz-Echevarría, L.; Andrade, F. Asymmetric dimethylarginine, endothelial dysfunction and renal disease. Int. J. Mol. Sci 2012, 13, 11288–11311. [Google Scholar]
- Abbasi, F.; Asagmi, T.; Cooke, J.P.; Lamendola, C.; McLaughlin, T.; Reaven, G.M.; Stühlinger, M.; Tsao, P.S. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am. J. Cardiol 2001, 88, 1201–1203. [Google Scholar]
- Ogawa, T.; Kimoto, M.; Sasaoka, K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J. Biol. Chem 1989, 264, 10205–10209. [Google Scholar]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol 2003, 23, 1455–1459. [Google Scholar]
- Vallance, P.; Leiper, J. Cardiovascular biology of the asymmetric dimethylarginine: Dimethylarginine dimethylaminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol 2004, 24, 1023–1030. [Google Scholar]
- Palm, F.; Onozato, M.L.; Luo, Z.; Wilcox, C.S. Dimethylarginine dimethylaminohydrolase (DDAH): Expression, regulation, and function in the cardiovascular and renal systems. Am. J. Physiol. Heart Circ. Physiol 2007, 293, H3227–H3245. [Google Scholar]
- Teerlink, T.; Luo, Z.; Palm, F.; Wilcox, C.S. Cellular ADMA: Regulation and action. Pharmacol. Res 2009, 60, 448–460. [Google Scholar]
- Böger, R.H.; Bode-Böger, S.M.; Szuba, A.; Tsao, P.S.; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric dimethylarginine (ADMA): A novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation 1998, 98, 1842–1847. [Google Scholar]
- Surdacki, A.; Nowicki, M.; Sandmann, J.; Tsikas, D.; Böger, R.H.; Bode-Böger, S.M.; Kruszelnicka-Kwiatkowska, O.; Kokot, F.; Dubiel, J.S.; Frölich, J.C. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J. Cardiovasc. Pharmacol 1999, 33, 652–658. [Google Scholar]
- Surdacki, A. L-arginine analogs—Inactive markers or active agents in atherogenesis? Cardiovasc. Hematol. Agents Med. Chem 2008, 6, 302–311. [Google Scholar]
- Gary, J.D.; Clarke, S. RNA and protein interactions modulated by protein arginine methylation. Prog. Nucleic. Acid Res. Mol. Biol 1998, 61, 65–131. [Google Scholar]
- Fickling, S.A.; Leone, A.M.; Nussey, S.S.; Vallance, P.; Whitley, G.St.J. Synthesis of NG, NG dimethylarginine by human endothelial cells. Endothelium 1993, 1, 137–140. [Google Scholar]
- Böger, R.H.; Bode-Böger, S.M.; Tsao, P.S.; Lin, P.S.; Chan, J.R.; Cooke, J.P. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J. Am. Coll. Cardiol 2000, 36, 2287–2295. [Google Scholar]
- Marliss, E.B.; Chevalier, S.; Gougeon, R.; Morais, J.A.; Lamarche, M.; Adegoke, O.A.; Wu, G. Elevations of plasma methylarginines in obesity and aging are related to insulin sensitivity and rates of protein turnover. Diabetologia 2006, 49, 351–359. [Google Scholar]
- Tutarel, O.; Denecke, A.; Bode-Böger, S.M.; Martens-Lobenhoffer, J.; Schieffer, B.; Westhoff-Bleck, M.; Kielstein, J.T. Symmetrical dimethylarginine outperforms CKD-EPI and MDRD-derived eGFR for the assessment of renal function in patients with adult congenital heart disease. Kidney Blood Press Res 2011, 34, 41–45. [Google Scholar]
- Martens-Lobenhoffer, J.; Westphal, S.; Awiszus, F.; Bode-Böger, S.M.; Luley, C. Determination of asymmetric dimethylarginine: Liquid chromatography-mass spectrometry or ELISA? Clin. Chem 2005, 51, 2188–2189. [Google Scholar]
- Valtonen, P.; Karppi, J.; Nyyssonen, K.; Valkonen, V.P.; Halonen, T.; Punnonen, K. Comparison of HPLC method and commercial ELISA assay for asymmetric dimethylarginine (ADMA) determination in human serum. J. Chromatogr. B 2005, 828, 97–102. [Google Scholar]
- Horowitz, J.D.; Heresztyn, T. An overview of plasma concentrations of asymmetric dimethylarginine (ADMA) in health and disease and in clinical studies: Methodological considerations. J. Chromatogr. B 2007, 851, 42–50. [Google Scholar]
- Miyazaki, H.; Matsuoka, H.; Cooke, J.P.; Usui, M.; Ueda, S.; Okuda, S.; Imaizumi, T. Endogenous nitric oxide synthase inhibitor: A novel marker of atherosclerosis. Circulation 1999, 99, 1141–1146. [Google Scholar]
- Ayer, J.G.; Harmer, J.A.; Nakhla, S.; Xuan, W.; Ng, M.K.; Raitakari, O.T.; Marks, G.B.; Celermajer, D.S. HDL-cholesterol, blood pressure, and asymmetric dimethylarginine are significantly associated with arterial wall thickness in children. Arterioscler. Thromb. Vasc. Biol 2009, 29, 943–949. [Google Scholar]
- Urbina, E.M.; Williams, R.V.; Alpert, B.S.; Collins, R.T.; Daniels, S.R.; Hayman, L.; Jacobson, M.; Mahoney, L.; Mietus-Snyder, M.; Rocchini, A.; et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: Recommendations for standard assessment for clinical research: A scientific statement from the American Heart Association. Hypertension 2009, 54, 919–950. [Google Scholar]
- Peake, M.; Whiting, M. Measurement of serum creatinine—Current status and future goals. Clin. Biochem. Rev 2006, 27, 173–184. [Google Scholar]
- Martens-Lobenhoffer, J.; Bode-Böger, S.M. Fast and efficient determination of arginine, symmetric dimethylarginine, and asymmetric dimethylarginine in biological fluids by hydrophilic-interaction liquid chromatography-electrospray tandem mass spectrometry. Clin. Chem 2006, 52, 488–493. [Google Scholar]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol 2009, 20, 629–637. [Google Scholar]
- Staples, A.; LeBlond, R.; Watkins, S.; Wong, C.; Brandt, J. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr. Nephrol 2010, 25, 2321–2326. [Google Scholar]
- Touboul, P.-J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Fatar, M.; et al. Mannheim carotid intima-media thickness consensus (2004–2006). Cerebrovasc. Dis 2007, 23, 75–80. [Google Scholar]

| Characteristic | |
|---|---|
| Age (years) | 10.1 ± 3.6 |
| Male gender (M/F) | 33/7 |
| Parental history of premature coronary artery disease | 10 (25%) |
| Height (percentiles) | 48 ± 28 |
| Weight (percentiles) | 43 ± 24 |
| Waist circumference (cm) | 63 (56–74) |
| Creatinine (μmol/L) | 40.2 (36.2–49.0) |
| Estimated glomerular filtration rate (mL/min per 1.73 m2) | 122.4 (109.7–136.1) |
| Low-density lipoproteins-cholesterol (mmol/L) | 2.2 (1.8–2.7) |
| High-density lipoproteins-cholesterol (mmol/L) | 1.5 (1.3–1.8) |
| Triglycerides (mmol/L) | 0.72 (0.54–0.94) |
| Glucose (mmol/L) | 4.6 (4.4–5.1) |
| Homocysteine (μmol/L) | 8.5 (7.4–10.1) |
| Averaged intima-media thickness of the common carotid artery (mm) | 0.45 (0.41–0.53) |
| Metabolite | Mean ± SD |
|---|---|
| l-arginine (μmol/L) | 69 ± 22 |
| ADMA (μmol/L) | 0.63 ± 0.12 |
| SDMA (μmol/L) | 0.56 ± 0.10 |
| SDMA/ADMA ratio | 0.91 ± 0.16 |
| eGFR | Ln (creatinine) | |
|---|---|---|
| ADMA | 0.19 | −0.22 |
| SDMA | −0.35 ** | 0.31 * |
| SDMA/ADMA ratio | −0.63 *** | 0.64 *** |
| l-arginine | 0.02 | −0.01 |
| Relationship | Mean standardized regression coefficient (β) ± SEM (p-values in parentheses) | ||
|---|---|---|---|
| Unadjusted | Age-adjusted | Height-adjusted | |
| SDMA vs. eGFR | −0.35 ± 0.15 (0.03) | −0.36 ± 0.18 (0.06) | −0.33 ± 0.18 (0.08) |
| SDMA/ADMA vs. eGFR | −0.63 ± 0.13 (<0.0001) | −0.52 ± 0.15 (0.001) | −0.52 ± 0.14 (0.0008) |
| SDMA vs. ln (creatinine) | 0.31 ± 0.15 (0.05) | 0.52 ± 0.27 (0.06) | 0.45 ± 0.28 (0.12) |
| SDMA/ADMA vs. ln (creat.) | 0.64 ± 0.12 (<0.0001) | 0.75 ± 0.22 (0.001) | 0.80 ± 0.22 (0.0009) |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jaźwińska-Kozuba, A.; Martens-Lobenhoffer, J.; Surdacki, A.; Kruszelnicka, O.; Rycaj, J.; Godula-Stuglik, U.; Bode-Böger, S.M. Associations between Endogenous Dimethylarginines and Renal Function in Healthy Children and Adolescents. Int. J. Mol. Sci. 2012, 13, 15464-15474. https://doi.org/10.3390/ijms131115464
Jaźwińska-Kozuba A, Martens-Lobenhoffer J, Surdacki A, Kruszelnicka O, Rycaj J, Godula-Stuglik U, Bode-Böger SM. Associations between Endogenous Dimethylarginines and Renal Function in Healthy Children and Adolescents. International Journal of Molecular Sciences. 2012; 13(11):15464-15474. https://doi.org/10.3390/ijms131115464
Chicago/Turabian StyleJaźwińska-Kozuba, Aleksandra, Jens Martens-Lobenhoffer, Andrzej Surdacki, Olga Kruszelnicka, Jarosław Rycaj, Urszula Godula-Stuglik, and Stefanie M. Bode-Böger. 2012. "Associations between Endogenous Dimethylarginines and Renal Function in Healthy Children and Adolescents" International Journal of Molecular Sciences 13, no. 11: 15464-15474. https://doi.org/10.3390/ijms131115464




