Microtubule Formation and Activities of Antioxidative Enzymes in PC12 Cells Exposed to Phosphatidylcholine Hydroperoxides
Abstract
:1. Introduction
2. Results
2.1. Inhibition of Microtubule Formation by PCOOH
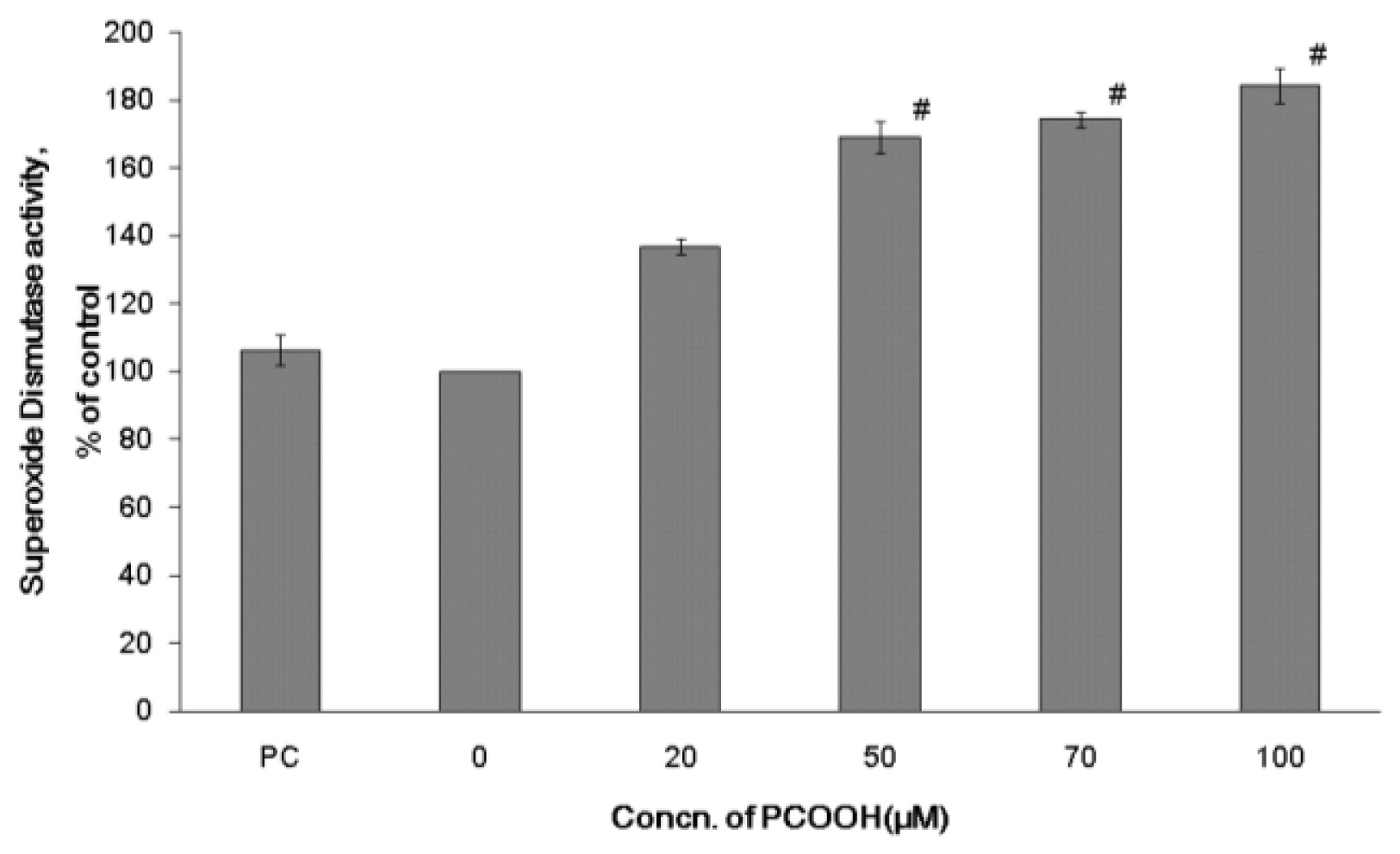
2.2. Effects of PCOOH on SOD Activities
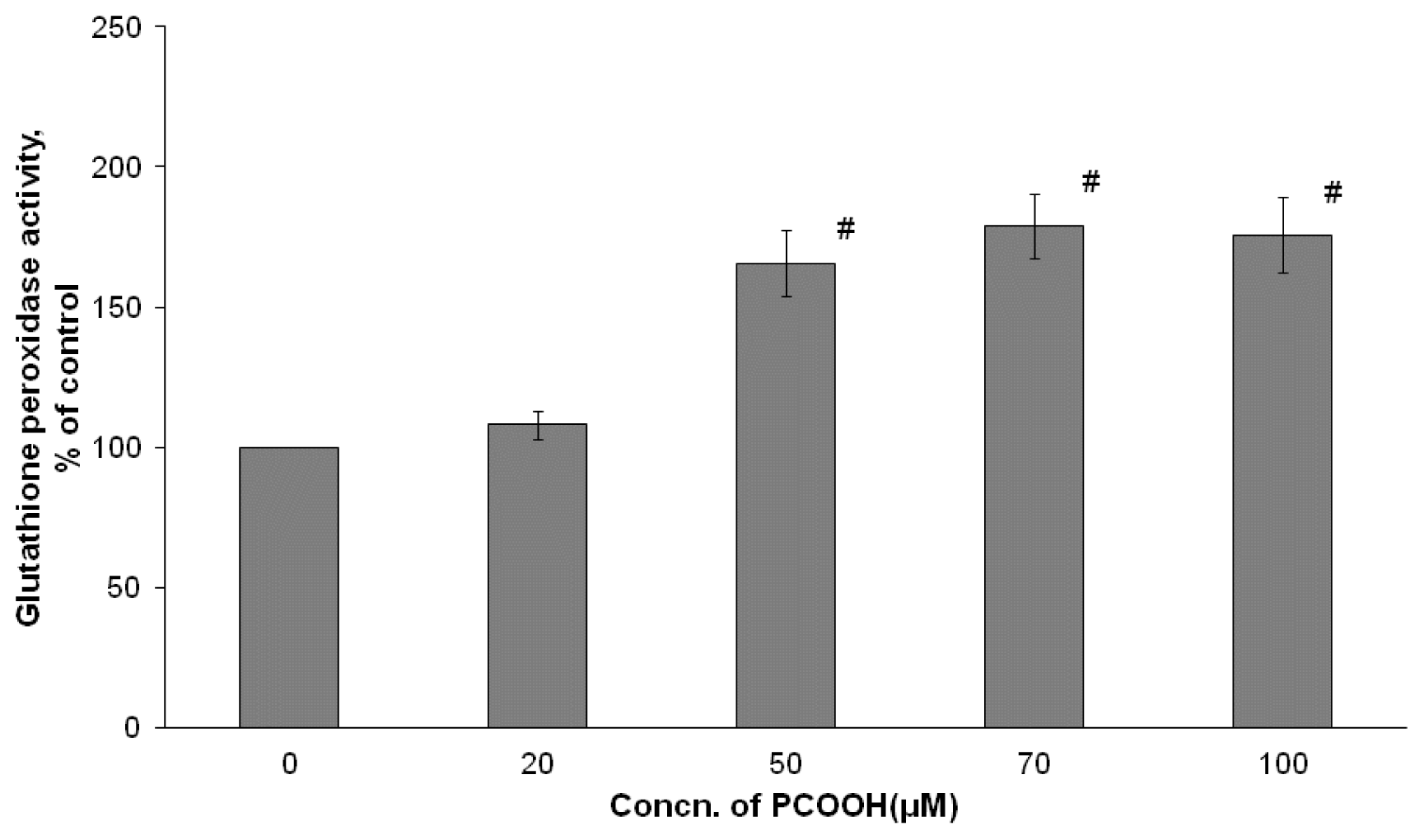
2.3. Effects of PCOOH on GPx Activities
2.4. Effects of PCOOH on CAT Activities
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Culture
4.3. Exposure of PC12 Cells to PCOOH
4.4. Assay of GTPase Activity
4.5. Antioxidant Enzymes
4.5.1. Determination of SOD Activity
4.5.2. Measurement of GPx Activities
4.5.3. Determination of CAT Activity
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Matsuyama, S.S.; Jarvik, L.F. Hypothesis: Microtubules, a key to Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 8152–8126. [Google Scholar]
- Masliah, E.; Terry, R.D.; DeTeresa, R.M.; Hansen, L.A. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci. Lett 1989, 103, 234–239. [Google Scholar]
- Iqbal, K.; Alonso, A.C.; Gong, C.X.; Khatoon, S.; Singh, T.J.; Grundke-Iqbal, I. Mechanism of neurofibrillary degeneration in Alzheimer’s disease. Mol. Neurobiol 1994, 9, 119–123. [Google Scholar]
- Kawakami, M.; Ward, L.; Doi, H. Inhibition of tubulin guanosine-5'-triphosphate by lipid peroxide: protective effects og vitamin A derivatives. J. Am. Oil Chem. Soc 1998, 75, 635–641. [Google Scholar]
- Yomogida, K.; Yoshida-Yamamoto, S.; Doi, H. Roles Microtubules in Maintenance of Nerve Cell Netwaorks; InTech: Rijeka, Croatia, 2011; pp. 35–50. [Google Scholar]
- Farooqui, A.A.; Horrocks, L.A. Plasmalogen-selective phospholipase A2 and its involvement in Alzheimer’s disease. Biochem. Soc. Trans 1998, 26, 243–246. [Google Scholar]
- Hajimohammadreza, I.; Brammer, M.J.; Eagger, S.; Burns, A.; Levy, R. Platelet and erythrocyte membrane changes in Alzheimer’s disease. Biochim. Biophys. Acta 1990, 1025, 208–214. [Google Scholar]
- Nakagawa, K.; Fujimoto, K.; Miyazawa, T. β-carotene as a high-potency antioxidant to prevent the formation of phospholipid hydroperoxides in red blood cells of mice. Biochim. Biophys. Acta 1996, 1299, 110–116. [Google Scholar]
- Pan, X.; Zhu, Y.; Lin, N.; Zhang, J.; Ye, Q.; Huang, H.; Chen, X. Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: Implications for Alzheimer’s disease. Mol. Neurodegener 2011, 30, 45. [Google Scholar]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother 2004, 58, 39–46. [Google Scholar]
- Christen, Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr 2000, 71, 621S–629S. [Google Scholar]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol 2010, 42, 1634–1650. [Google Scholar]
- Bhor, V.M.; Raghuram, N.; Sivakami, S. Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int. J. Biochem. Cell Biol 2004, 36, 89–97. [Google Scholar]
- Kakkar, R.; Kalra, J.; Mantha, S.V.; Prasad, K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell. Biochem 1995, 151, 113–119. [Google Scholar]
- Kawakami, M.; Ward, L.; Doi, H. Mechanisms of tubulin modification by phosphatidylcholine hydroperoxides. Lipids 2000, 35, 205–211. [Google Scholar]
- Mueller, H.W.; Purdon, A.D.; Smith, J.B.; Wykle, R.L. 1-O-alkyl-linked phosphoglycerides of human platelets: distribution of arachidonate and other acyl residues in the ether-linked and diacyl species. Lipids 1983, 18, 814–819. [Google Scholar]
- Siqueira, I.R.; Fochesatto, C.; de Andrade, A.; Santos, M.; Hagen, M.; Bello-Klein, A.; Netto, C.A. Total antioxidant capacity is impaired in different structures from aged rat brain. Int. J. Dev. Neurosci 2005, 23, 663–671. [Google Scholar]
- Yamanaka, Y.; Yoshida, S.; Doi, H. NGF-induced neurite outgrowth of PC12 cells in the presence of phosphatidylcholine hydroperoxides: Implication for ageing. Mech. Ageing Dev 2008, 129, 215–222. [Google Scholar]
- O'Brien, E.T.; Voter, W.A.; Erickson, H.P. GTP hydrolysis during microtubule assembly. Biochemistry 1987, 26, 4148–4156. [Google Scholar]
- Seckler, R.; Wu, G.M.; Timasheff, S.N. Interactions of tubulin with guanylyl-(beta-gamma-methylene)diphosphonate. Formation and assembly of a stoichiometric complex. J. Biol. Chem 1990, 265, 7655–7661. [Google Scholar]
- Doi, H.; Imanishi, T.; Iwami, K.; Ibuki, F. Is microtubule assembly not associated with GTP hydrolysis? Agric. Biol. Chem 1991, 55, 245–246. [Google Scholar]
- Benzi, G.; Marzatico, F.; Pastoris, O.; Villa, R.F. Influence of oxidative stress on the age-linked alterations of the cerebral glutathione system. J. Neurosci. Res 1990, 26, 120–128. [Google Scholar]
- Ochiai, T.; Soeda, S.; Ohno, S.; Tanaka, H.; Shoyama, Y.; Shimeno, H. Crocin prevents the death of PC-12 cells through sphingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem. Int 2004, 44, 321–330. [Google Scholar]
- Ferrari, R.; Agnoletti, L.; Comini, L.; Gaia, G.; Bachetti, T.; Cargnoni, A.; Ceconi, C.; Curello, S.; Visioli, O. Oxidative stress during myocardial ischaemia and heart failure. Eur. Heart J 1998, 19, B2–B11. [Google Scholar]
- Halliwell, B. Oxidants and the central nervous system: Some fundamental questions. Is oxidant damage relevant to Parkinson’s disease, Alzheimer’s disease, traumatic injury or stroke? Acta Neurol. Scand. Suppl 1989, 126, 23–33. [Google Scholar]
- Imre, S.; Juhasz, E. The effect of oxidative stress on inbred mice of different ages. Mech. Ageing Dev 1987, 38, 259–266. [Google Scholar]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol 1956, 11, 298–300. [Google Scholar]
- Barja, G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radic. Biol. Med 2002, 33, 1167–1172. [Google Scholar]
- Muscari, C.; Giaccari, A.; Giordano, E.; Clo, C.; Guarnieri, C.; Caldarera, C.M. Role of reactive oxygen species in cardiovascular aging. Mol. Cell. Biochem. 1996, 160–161, 159–166. [Google Scholar]
- Wickens, A.P. Ageing and the free radical theory. Respir. Physiol 2001, 128, 379–391. [Google Scholar]
- Pansarasa, O.; Bertorelli, L.; Vecchiet, J.; Felzani, G.; Marzatico, F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic. Biol. Med 1999, 27, 617–622. [Google Scholar]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol 2003, 15, 247–254. [Google Scholar]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev 1998, 78, 547–581. [Google Scholar]
- Kedar, N.P. Can we prevent Parkinson’s and Alzheimer’s disease? J. Postgrad. Med 2003, 49, 236–245. [Google Scholar]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med 2008, 45, 443–452. [Google Scholar]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem 2006, 97, 1634–1658. [Google Scholar]
- Miyazawa, T.; Suzuki, T.; Fujimoto, K.; Kinoshita, M. Age-related change of phosphatidylcholine hydroperoxide and phosphatidylethanolamine hydroperoxide levels in normal human red blood cells. Mech. Ageing Dev 1996, 86, 145–150. [Google Scholar]
- Miquel, J.; Ramirez-Bosca, A.; Soler, A.; Diez, A.; Carrion-Gutierrez, M.A.; Diaz-Alperi, J.; Quintanilla-Ripoll, E.; Bernd, A.; Quintanilla-Almagro, E. Increase with age of serum lipid peroxides: Implications for the prevention of atherosclerosis. Mech. Ageing Dev 1998, 100, 17–24. [Google Scholar]
- Kinoshita, M.; Oikawa, S.; Hayasaka, K.; Sekikawa, A.; Nagashima, T.; Toyota, T.; Miyazawa, T. Age-related increases in plasma phosphatidylcholine hydroperoxide concentrations in control subjects and patients with hyperlipidemia. Clin. Chem 2000, 46, 822–828. [Google Scholar]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar]
- Kolla, N.; Wei, Z.; Richardson, J.S.; Li, X.M. Amitriptyline and fluoxetine protect PC12 cells from cell death induced by hydrogen peroxide. J. Psychiatry Neurosci 2005, 30, 196–201. [Google Scholar]
- Morikawa, K.; Shimokawa, H.; Matoba, T.; Kubota, H.; Akaike, T.; Talukder, M.A.; Hatanaka, M.; Fujiki, T.; Maeda, H.; Takahashi, S.; et al. Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J. Clin. Invest 2003, 112, 1871–1879. [Google Scholar]
- Wheeler, C.R.; Salzman, J.A.; Elsayed, N.M.; Omaye, S.T.; Korte, D.W., Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990, 184, 193–199. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamanaka, Y.; Yoshida-Yamamoto, S.; Doi, H. Microtubule Formation and Activities of Antioxidative Enzymes in PC12 Cells Exposed to Phosphatidylcholine Hydroperoxides. Int. J. Mol. Sci. 2012, 13, 15510-15522. https://doi.org/10.3390/ijms131215510
Yamanaka Y, Yoshida-Yamamoto S, Doi H. Microtubule Formation and Activities of Antioxidative Enzymes in PC12 Cells Exposed to Phosphatidylcholine Hydroperoxides. International Journal of Molecular Sciences. 2012; 13(12):15510-15522. https://doi.org/10.3390/ijms131215510
Chicago/Turabian StyleYamanaka, Yukako, Shumi Yoshida-Yamamoto, and Hiroshi Doi. 2012. "Microtubule Formation and Activities of Antioxidative Enzymes in PC12 Cells Exposed to Phosphatidylcholine Hydroperoxides" International Journal of Molecular Sciences 13, no. 12: 15510-15522. https://doi.org/10.3390/ijms131215510



