Activation Energy of Aggregation-Disaggregation Self-Oscillation of Polymer Chain
Abstract
:1. Introduction
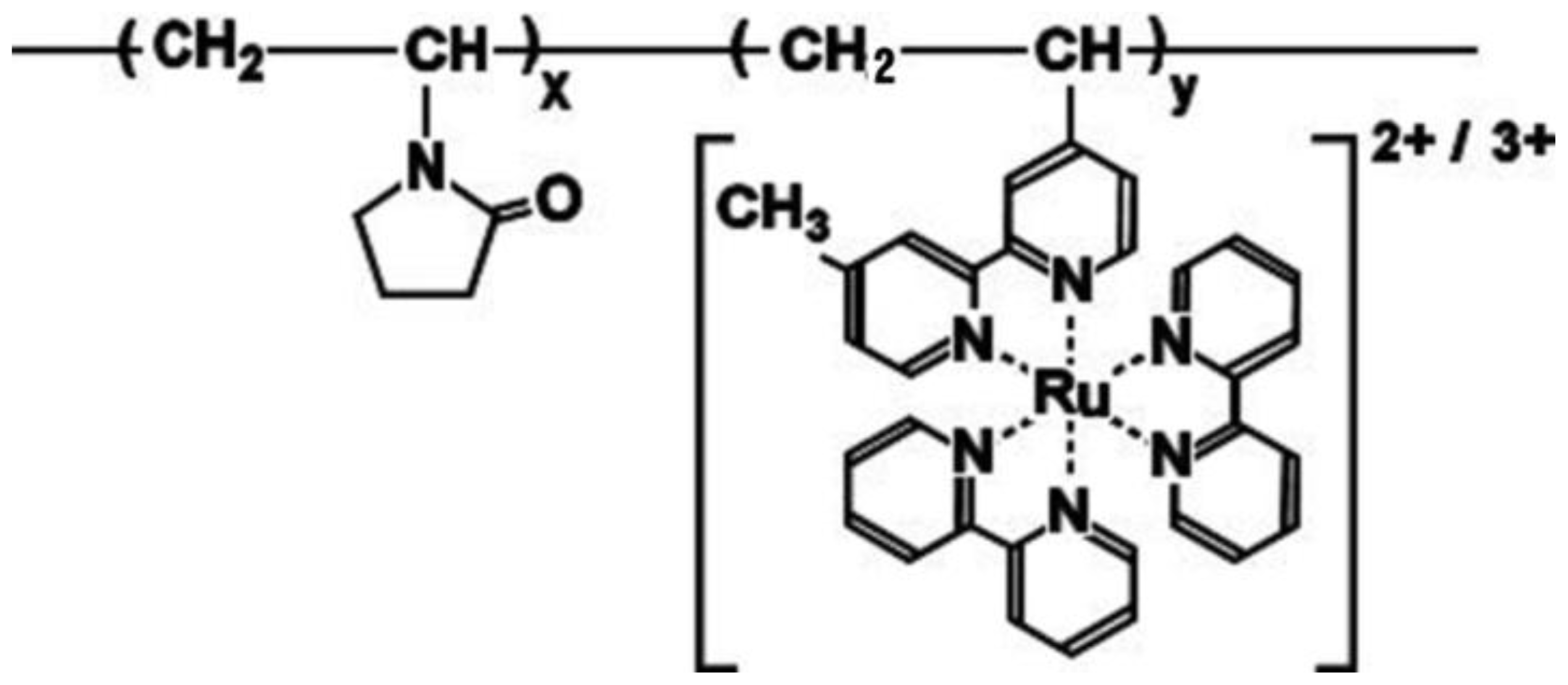
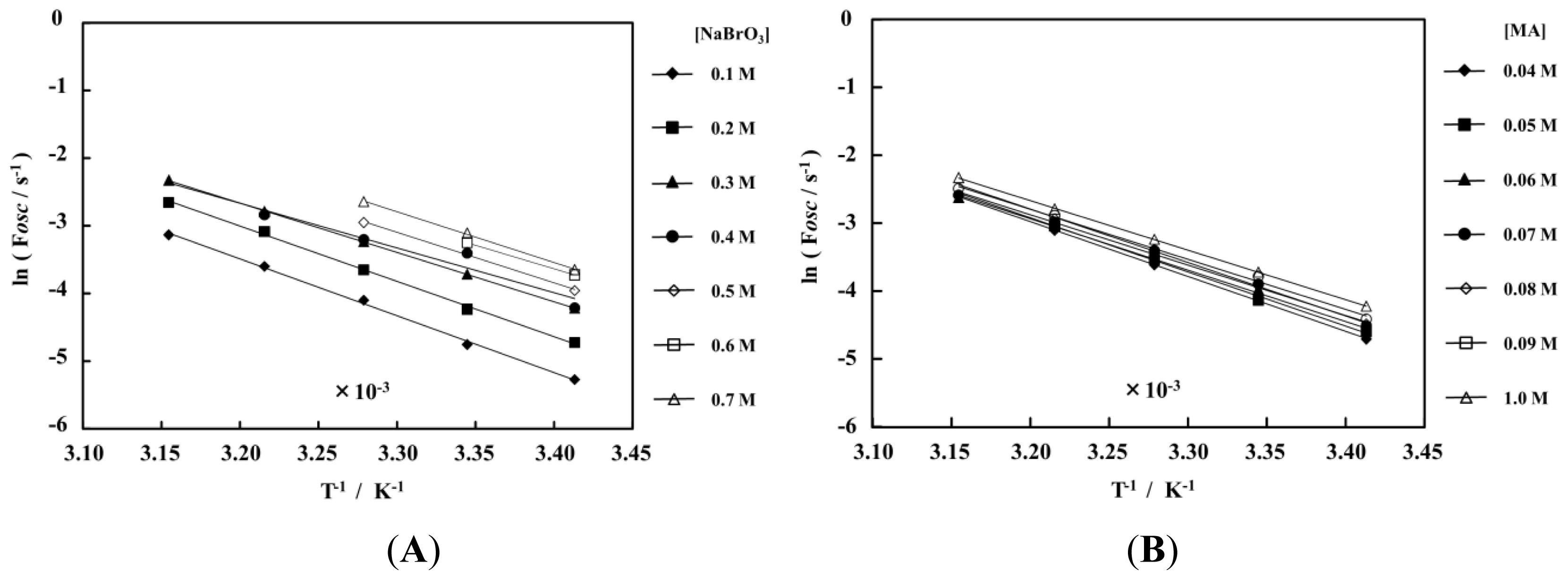
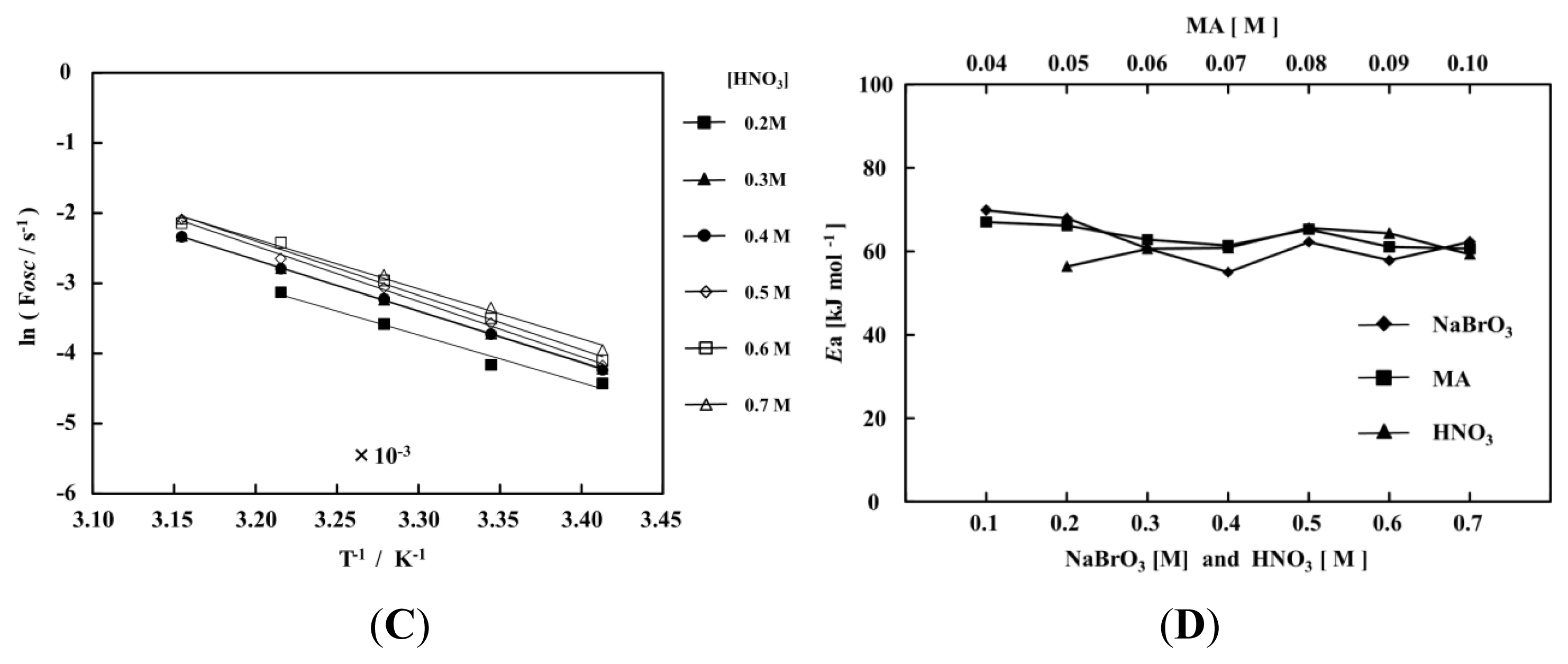
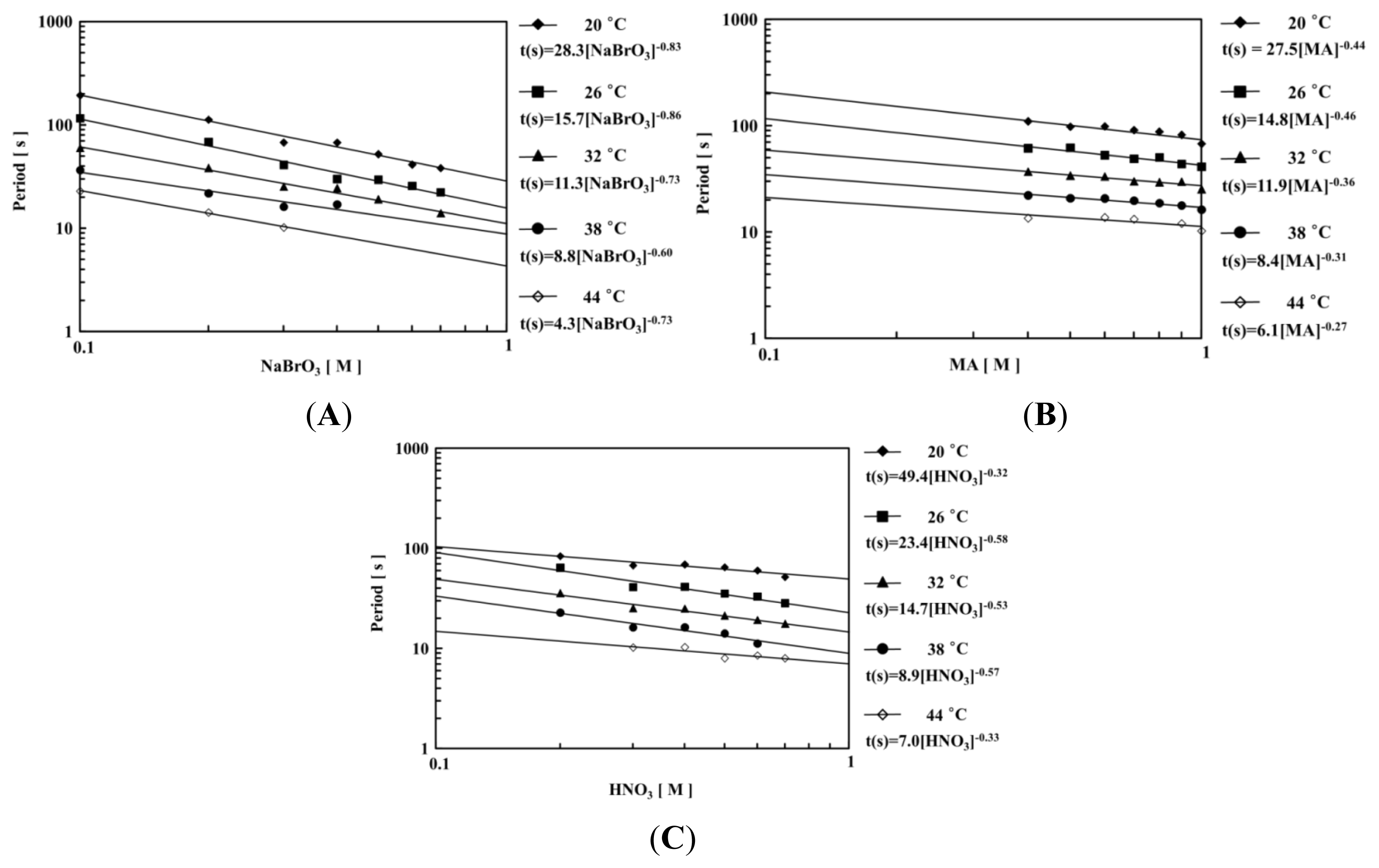
2. Results and Discussion
3. Materials and Methods
3.1. Polymerization
3.2. Measurement of Optical Transmittance Self-Oscillations
4. Conclutions
Acknowledgments
References
- Zaikin, A.N.; Zhabotinsky, A.M. Concentration wave propagation in two-dimensional liquid-phase self-oscillating system. Nature 1970, 225, 535–537. [Google Scholar]
- Reusser, E.J.; Field, R.J. The transition from phase waves to trigger waves in a model of the Zhabotinskii reaction. J. Am. Chem. Soc 1979, 101, 1063–1071. [Google Scholar]
- Scott, S.K. Chemical Chaos, 1st ed; Oxford University Press: Oxford, UK; p. 1991.
- Field, R.J.; Burger, M. Oscillations and Traveling Waves in Chemical Systems; John Wiley & Sons: New York, NY, USA; p. 1985.
- Nicolis, G.; Prigogine, I. Self Orgainization in Nonequilibrium Systems; Wiley: New York, NY, USA; p. 1977.
- Murray, J.D. Mathematical Biology; Springer-Verlag: Berlin, Germany; p. 1990.
- Mori, H.; Kuramoto, Y. Dissipative Structures and Chaos; Springer-Verlag: Berlin, Germany; p. 1997.
- Amemiya, T.; Ohmori, T.; Yamaguchi, T. An oregonator-class model for photoinduced behavior in the Ru (bpy) 32+-catalyzed Belousov-Zhabotinsky reaction. J. Phys. Chem. A 2000, 104, 336–344. [Google Scholar]
- Ishiwatari, T.; Kawaguchi, M.; Mitsuishi, M. Oscillatry reactions in polymer systems. J. Polymer Sci. A Polymer Chem 1984, 22, 2699–2704. [Google Scholar]
- Yoshida, R.; Sakai, T.; Ito, S.; Yamaguchi, T. Self-oscillation of polymer chains with rhythmical soluble-insoluble changes. J. Am. Chem. Sci 2002, 124, 8095–8098. [Google Scholar]
- Yoshida, R.; Takahashi, T.; Yamaguchi, T.; Ichijo, H. Self-oscillating gel. J. Am. Chem. Soc 1996, 118, 5134–5135. [Google Scholar]
- Hara, Y.; Yoshida, R. Self-oscillating polymer fueled by organic acid. J. Phys. Chem. B 2008, 112, 8427–8429. [Google Scholar]
- Hara, Y.; Yoshida, R. Self-oscillation of polymer chains induced by the Belousov-Zhabotinsky reaction under acid-free conditions. J. Phys. Chem. B 2005, 109, 9451–9454. [Google Scholar]
- Hara, Y.; Yoshida, R. Damping behavior of aggregation-disaggregation self-oscillation for a polymer chain. Marcomol. Rapid Commun 2009, 30, 1656–1662. [Google Scholar]
- Hara, Y.; Yoshida, R. Control of oscillating behavior for the self-oscillating polymer with pH-control site. Langmuir 2005, 21, 9773–9776. [Google Scholar]
- Hara, Y.; Yoshida, R. A viscosity self-oscillation of polymer solution induced by the BZ reaction under acid-free condition. J. Chem. Phys 2008, 128, 224904. [Google Scholar]
- Maeda, S.; Hara, Y.; Sakai, T.; Yoshida, R.; Hashimoto, S. Self-walking gel. Adv. Mater. 2007, 19, 3480–3484. [Google Scholar]
- Maeda, S.; Hara, Y.; Yoshida, R.; Hashimoto, S. Control of the dynamic motion of a gel actuator driven by the Belousov-Zhabotinsky reaction. Macromol. Rapid. Commun 2008, 29, 401–405. [Google Scholar]
- Maeda, S.; Hara, Y.; Yoshida, R.; Hashimoto, S. Peristaltic motion of polymer gels. Angew. Chem. Int. Ed 2008, 47, 6690–6693. [Google Scholar]
- Hara, Y.; Maeda, S.; Hashimoto, S.; Yoshida, R. Molecular design and functional control of novel self-oscillating polymers. Int. J. Mol. Sci 2010, 11, 704–718. [Google Scholar]
- Hara, Y.; Jahan, R.A. Influence of initial substrate concentration of the Belouzov-Zhabotinsky reaction on transmittance self-oscillation for a nonthermoresponsive polymer chain. Polymers 2011, 3, 330–339. [Google Scholar]
- Hara, Y. Trasmittance self-oscillating behavior of a non-thermoresponsive polymer chain induced by the Belouzov-Zhabotinsky (BZ) reaction. Key Eng. Mater. 2011, 480–481, 369–374. [Google Scholar]
- Hara, Y. Effect of substrate concentrations of the BZ reaction on period of self-oscillation for non-thermoresponsive polymer chain. Key Eng. Mater. 2011, 480–481, 357–362. [Google Scholar]
- Miyakawa, K.; Sakamoto, F.; Yoshida, R.; Yamaguchi, T.; Kokufuta, E. Chemical waves in self-oscillating gels. Phys. Rev. E 2000, 62, 793–798. [Google Scholar]
- Kuhnert, L.; Krug, H.J. Kinetics of chemical waves in the acidic bromate-malonic acid-tris(bipyridine)ruthenium(2+) system in comparison with the ferroin system. J. Phys. Chem 1987, 91, 730–733. [Google Scholar]
- Nakamaru, S.; Maeda, S.; Hara, Y.; Hashimoto, S. Control of autonomous swelling-deswelling behavior for a polymer gel. J. Phys. Chem. B 2009, 113, 4609–4613. [Google Scholar]
- Field, R.J.; Koros, E.; Noyes, R.M. Oscillations in chemical systems. II. Thorough analysis of temporal oscillation in the bromate-cerium-malonic acid system. J. Am. Chem. Soc 1972, 94, 8649–8664. [Google Scholar]
- Field, R.J.; Noyes, R.M. Oscillations in chemical systems. IV. Limit cycle behavior in a model of a real chemical reaction. J. Chem. Phys 1974, 60, 1877–1884. [Google Scholar]
- Gyorgyi, L.; Turanyi, T.; Field, R.J. Mechanistic details of the oscillatory Belousov-Zhabotinskii reaction. J. Chem. Phys 1990, 94, 7162–7170. [Google Scholar]
- Turanyi, T.; Gyorgyi, L.; Field, R.J. Analysis and simplification of the GTF model of the Belousov-Zhabotinsky reaction. J. Chem. Phys 1993, 97, 1931–1941. [Google Scholar]
- Wood, P.M.; Ross, J. A quantitative study of chemical waves in the Belousov-Zhabotinsky reaction. J. Chem. Phys 1985, 82, 1924. [Google Scholar]
- Tyson, J.J.; Fife, P.C. Target patterns in a realistic model of the Belousov-Zhabotinskii reaction. J. Chem. Phys 1980, 73, 2224. [Google Scholar]
- Yoshida, R.; Tanaka, M.; Onodera, S.; Yamaguchi, T.; Kokufuta, E. In-phase synchronization of chemical and mechanical oscillations in self-oscillating gels. J. Phys. Chem. A 2000, 104, 7549–7555. [Google Scholar]

| Temperature | Concentration of NaBrO3 | ||||||
|---|---|---|---|---|---|---|---|
| 0.1 M | 0.2 M | 0.3 M | 0.4 M | 0.5 M | 0.6 M | 0.7 M | |
| 20 °C | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| 26 °C | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| 32 °C | ○ | ○ | ○ | ○ | ○ | × | ○ |
| 38 °C | ○ | ○ | ○ | ○ | × | × | × |
| 44 °C | ○ | ○ | ○ | × | × | × | × |
| Temperature | Concentration of MA | ||||||
|---|---|---|---|---|---|---|---|
| 0.04 M | 0.05 M | 0.06 M | 0.07 M | 0.08 M | 0.09 M | 0.10 M | |
| 20 °C | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| 26 °C | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| 32 °C | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| 38 °C | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| 44 °C | ○ | × | ○ | ○ | × | ○ | ○ |
| Temperature | Concentration of MA | ||||||
|---|---|---|---|---|---|---|---|
| 0.1 M | 0.2 M | 0.3 M | 0.4 M | 0.5 M | 0.6 M | 0.7 M | |
| 20 °C | × | ○ | ○ | ○ | ○ | ○ | ○ |
| 26 °C | × | ○ | ○ | ○ | ○ | ○ | ○ |
| 32 °C | × | ○ | ○ | ○ | ○ | ○ | ○ |
| 38 °C | × | ○ | ○ | ○ | ○ | ○ | × |
| 44 °C | × | × | ○ | ○ | ○ | ○ | ○ |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hara, Y.; Jahan, R.A. Activation Energy of Aggregation-Disaggregation Self-Oscillation of Polymer Chain. Int. J. Mol. Sci. 2012, 13, 16281-16290. https://doi.org/10.3390/ijms131216281
Hara Y, Jahan RA. Activation Energy of Aggregation-Disaggregation Self-Oscillation of Polymer Chain. International Journal of Molecular Sciences. 2012; 13(12):16281-16290. https://doi.org/10.3390/ijms131216281
Chicago/Turabian StyleHara, Yusuke, and Rumana A. Jahan. 2012. "Activation Energy of Aggregation-Disaggregation Self-Oscillation of Polymer Chain" International Journal of Molecular Sciences 13, no. 12: 16281-16290. https://doi.org/10.3390/ijms131216281
APA StyleHara, Y., & Jahan, R. A. (2012). Activation Energy of Aggregation-Disaggregation Self-Oscillation of Polymer Chain. International Journal of Molecular Sciences, 13(12), 16281-16290. https://doi.org/10.3390/ijms131216281




