1-Benzyl-2-Phenylbenzimidazole (BPB), a Benzimidazole Derivative, Induces Cell Apoptosis in Human Chondrosarcoma through Intrinsic and Extrinsic Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
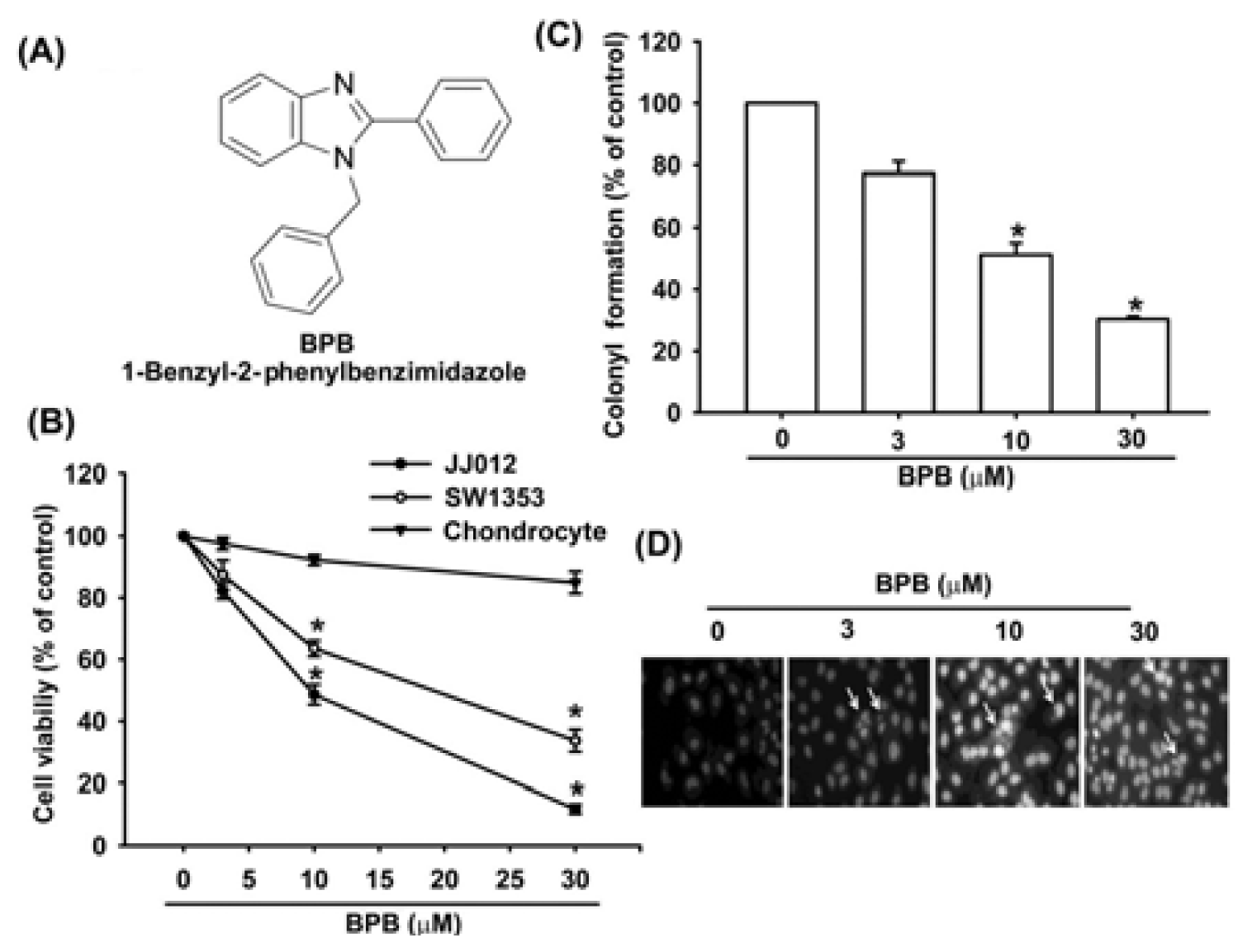
2.3. MTT Assay
2.4. Colony Assay
2.5. DAPI Staining
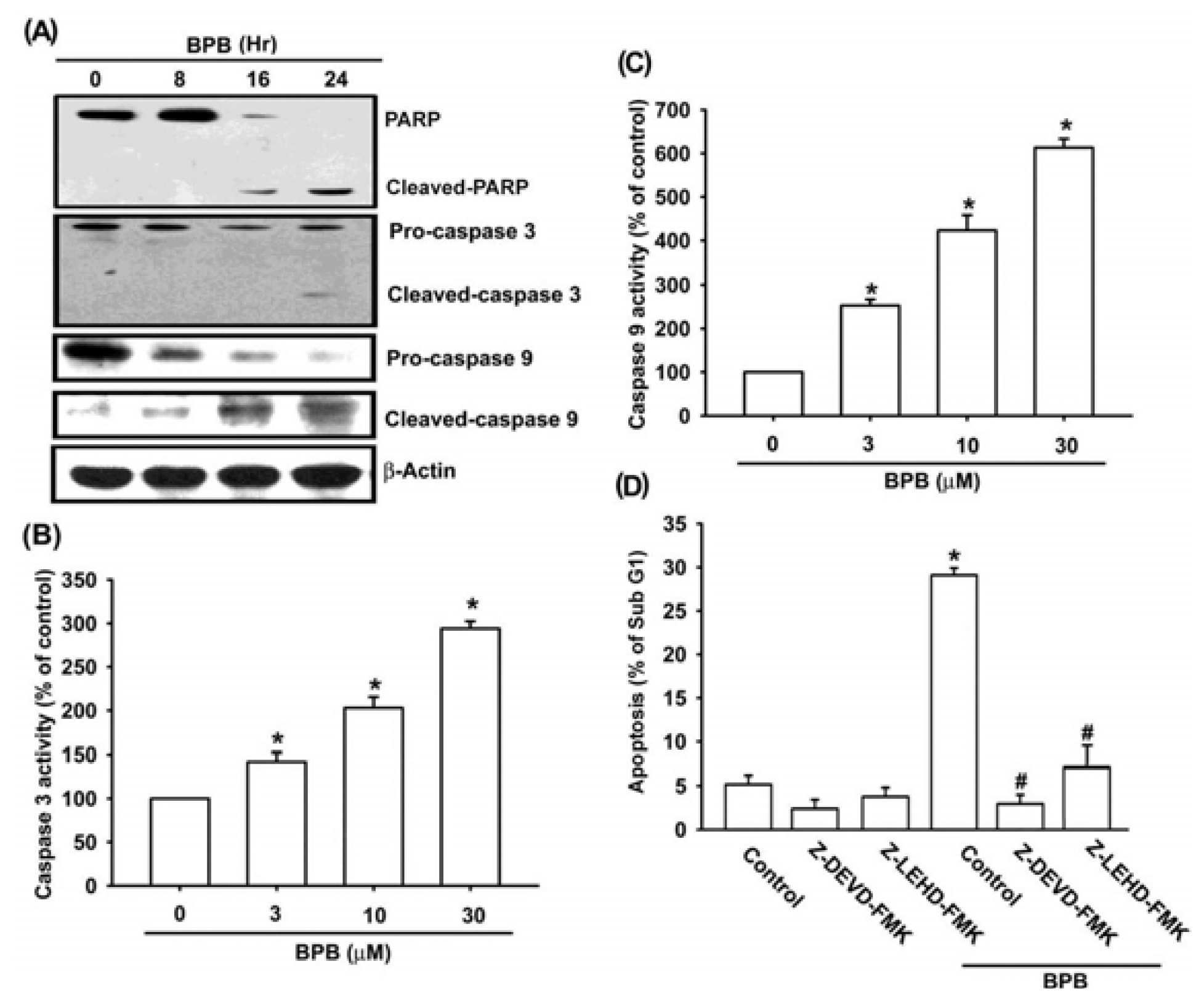
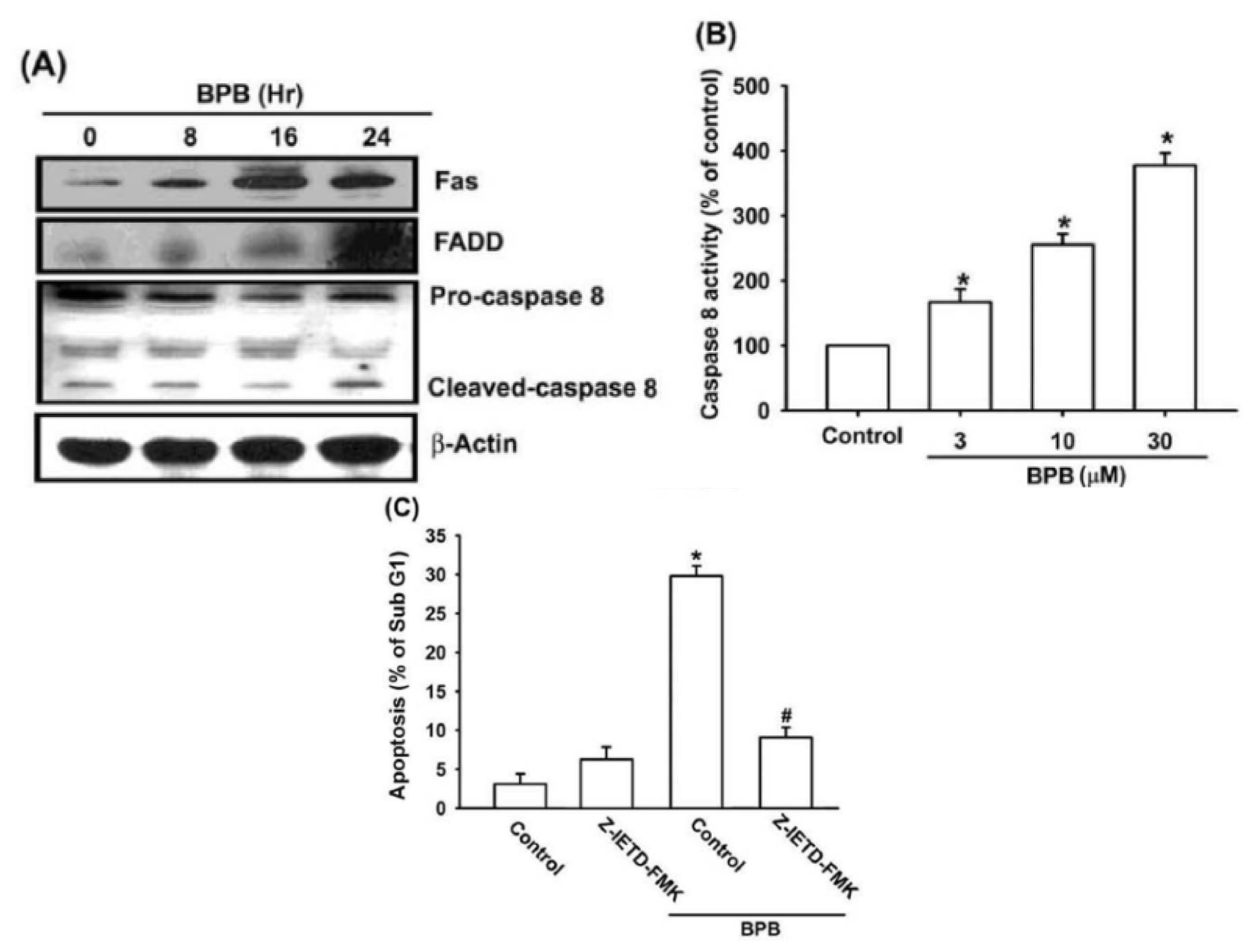
2.6. Quantification of Apoptosis by Flow Cytometry
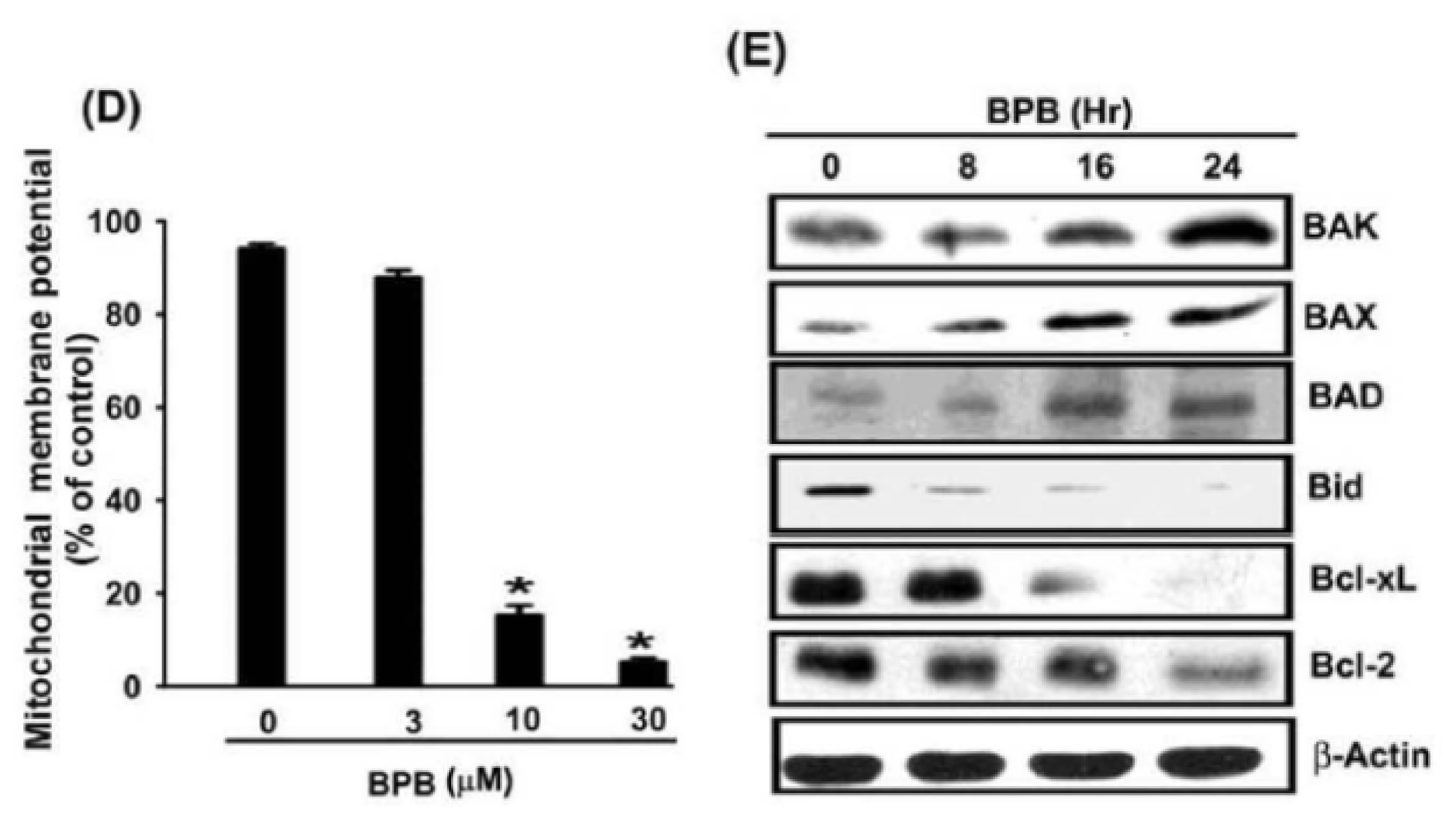
2.7. Determination of Mitochondrial Membrane Potential
2.8. Western Blot Analysis
2.9. Caspase Activity Assay
2.10. siRNA Transfection
2.11. In vivo Tumor Xenograft Study
2.12. Statistics
3. Results and Discussion
3.1. BPB Induces Cell Apoptosis in Human Chondrosarcoma Cells
3.2. Intrinsic and Extrinsic Pathways Are Mediates BPB-Induced Cell Apoptosis in Human Chondrosarcoma Cells
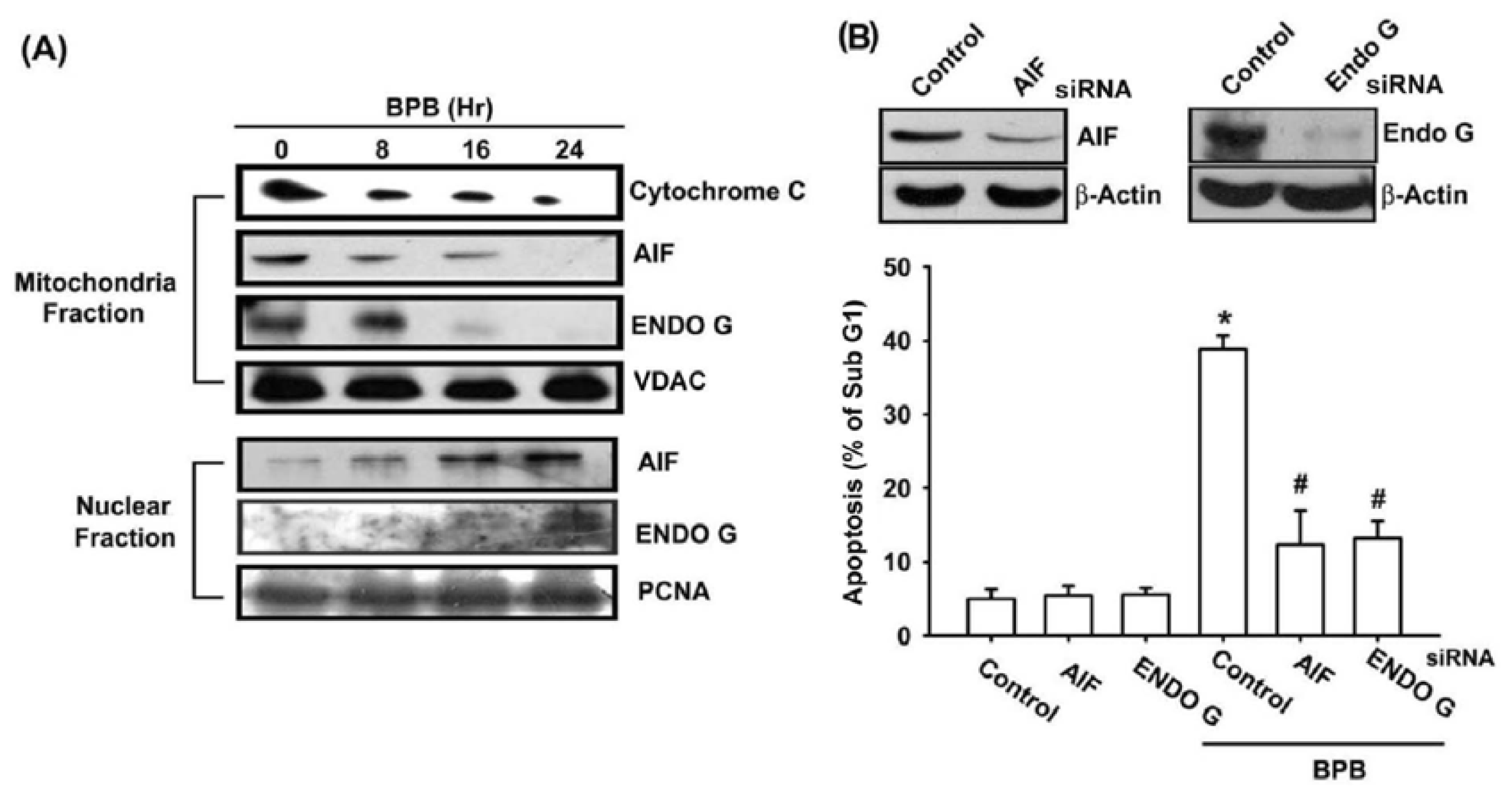
3.3. BPB Increases Cytochrome c, AIF, and Endo G Release in Chondrosarcoma Cells
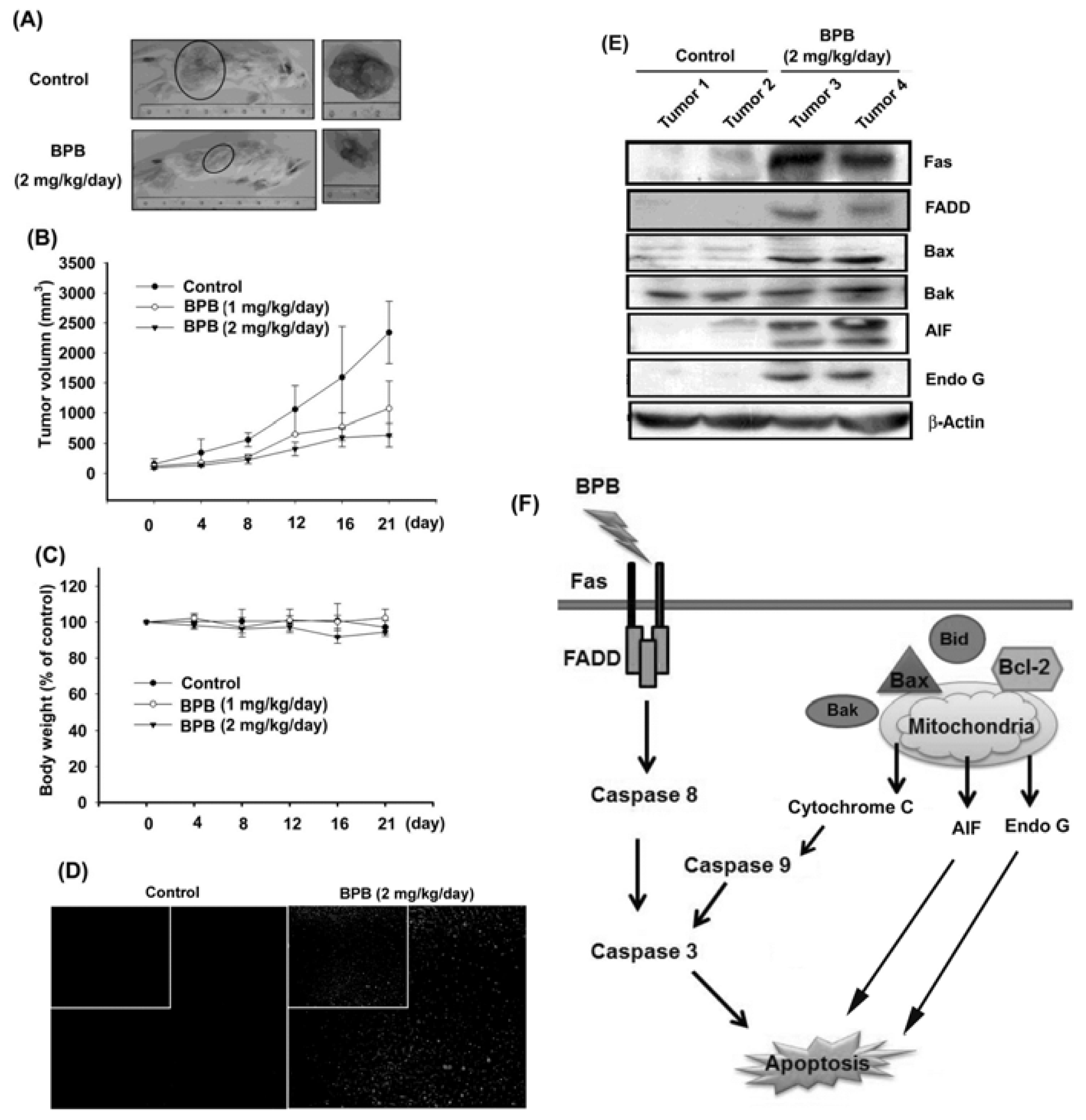
3.4. BPB Inhibits Tumor Growth in the Mouse Xenograft Model of JJ012 Cells
4. Discussion and Conclusions
Acknowledgments
- Conflict of InterestAll authors have no financial or personal relationships with other people or organizations that could inappropriately influence our work.
References
- Kato, T.; Tohnosu, N.; Suwa, T.; Takahashi, H.; Tokuizumi, M.; Uehara, T.; Kobayashi, T.K. Metaplastic breast carcinoma with chondrosarcomatous differentiation: Fine-needle aspiration cytology findings. A case report. Diagn. Cytopathol 2006, 34, 772–775. [Google Scholar]
- Yuan, J.; Dutton, C.M.; Scully, S.P. RNAi mediated MMP-1 silencing inhibits human chondrosarcoma invasion. J. Orthop. Res 2005, 23, 1467–1474. [Google Scholar]
- Taatjes, D.J.; Wadsworth, M.P.; Quinn, A.S.; Rand, J.H.; Bovill, E.G.; Sobel, B.E. Imaging aspects of cardiovascular disease at the cell and molecular level. Histochem. Cell Biol 2008, 130, 235–245. [Google Scholar]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol 2008, 9, 231–241. [Google Scholar]
- Grutter, M.G. Caspases: Key players in programmed cell death. Curr. Opin. Struct. Biol 2000, 10, 649–655. [Google Scholar]
- Tsujimoto, Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J. Cell. Physiol 2003, 195, 158–167. [Google Scholar]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol 1999, 15, 269–290. [Google Scholar]
- Penninger, J.M.; Kroemer, G. Mitochondria, AIF and caspases—Rivaling for cell death execution. Nat. Cell Biol 2003, 5, 97–99. [Google Scholar]
- Arnoult, D.; Gaume, B.; Karbowski, M.; Sharpe, J.C.; Cecconi, F.; Youle, R.J. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J 2003, 22, 4385–4399. [Google Scholar]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem 2012, 20, 6208–6236. [Google Scholar]
- Beaulieu, P.L.; Bousquet, Y.; Gauthier, J.; Gillard, J.; Marquis, M.; McKercher, G.; Pellerin, C.; Valois, S.; Kukolj, G. Non-nucleoside benzimidazole-based allosteric inhibitors of the hepatitis C virus NS5B polymerase: Inhibition of subgenomic hepatitis C virus RNA replicons in Huh-7 cells. J. Med. Chem 2004, 47, 6884–6892. [Google Scholar]
- Sachs, G.; Shin, J.M.; Hunt, R. Novel approaches to inhibition of gastric acid secretion. Curr. Gastroenterol. Rep 2010, 12, 437–447. [Google Scholar]
- Tsukamoto, G.; Yoshino, K.; Kohno, T.; Ohtaka, H.; Kagaya, H.; Ito, K. Synthesis and antiinflammatory activity of some 2-(substituted-pyridinyl)benzimidazoles. J. Med. Chem 1980, 23, 734–738. [Google Scholar]
- Ayhan-Kilcigil, G.; Kus, C.; Ozdamar, E.D.; Can-Eke, B.; Iscan, M. Synthesis and antioxidant capacities of some new benzimidazole derivatives. Arch. Pharm. (Weinheim) 2007, 340, 607–611. [Google Scholar]
- Cheng, J.; Xie, J.; Luo, X. Synthesis and antiviral activity against Coxsackie virus B3 of some novel benzimidazole derivatives. Bioorg. Med. Chem. Lett 2005, 15, 267–269. [Google Scholar]
- He, Y.; Yang, J.; Wu, B.; Risen, L.; Swayze, E.E. Synthesis and biological evaluations of novel benzimidazoles as potential antibacterial agents. Bioorg. Med. Chem. Lett 2004, 14, 1217–1220. [Google Scholar]
- Ibrahim, E.S.; Omar, A.M.; Khalil, M.A. Novel potential anticancer agents derived from benzimidazole. J. Pharm. Sci 1980, 69, 1348–1350. [Google Scholar]
- Liu, J.F.; Chang, C.S.; Fong, Y.C.; Kuo, S.C.; Tang, C.H. FPipTB, a benzimidazole derivative, induces chondrosarcoma cell apoptosis via endoplasmic reticulum stress and apoptosis signal-regulating kinase 1. Mol. Carcinog. 2011, 51. [Google Scholar] [CrossRef]
- Li, T.M.; Lin, T.Y.; Hsu, S.F.; Wu, C.M.; Su, Y.C.; Kao, S.T.; Chang, C.S.; Fong, Y.C.; Tang, C.H. The novel benzimidazole derivative, MPTB, induces cell apoptosis in human chondrosarcoma cells. Mol. Carcinog 2011, 50, 791–803. [Google Scholar]
- Scully, S.P.; Berend, K.R.; Toth, A.; Qi, W.N.; Qi, Z.; Block, J.A. Marshall Urist Award. Interstitial collagenase gene expression correlates with in vitro invasion in human chondrosarcoma. Clin. Orthop. Relat. Res 2000, 376, 291–303. [Google Scholar]
- Sung, L.Y.; Lo, W.H.; Chiu, H.Y.; Chen, H.C.; Chung, C.K.; Lee, H.P.; Hu, Y.C. Modulation of chondrocyte phenotype via baculovirus-mediated growth factor expression. Biomaterials 2007, 28, 3437–3447. [Google Scholar]
- Tang, C.H.; Hsu, C.J.; Fong, Y.C. The CCL5/CCR5 axis promotes interleukin-6 production in human synovial fibroblasts. Arthritis. Rheum 2010, 62, 3615–3624. [Google Scholar]
- Huang, W.W.; Chiu, Y.J.; Fan, M.J.; Lu, H.F.; Yeh, H.F.; Li, K.H.; Chen, P.Y.; Chung, J.G.; Yang, J.S. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol. Nutr. Food Res 2010, 54, 1585–1595. [Google Scholar]
- Dijkers, P.F.; Birkenkamp, K.U.; Lam, E.W.; Thomas, N.S.; Lammers, J.W.; Koenderman, L.; Coffer, P.J. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: Protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol 2002, 156, 531–542. [Google Scholar]
- Chen, J.T.; Fong, Y.C.; Li, T.M.; Liu, J.F.; Hsu, C.W.; Chang, C.S.; Tang, C.H. DDTD, an isoflavone derivative, induces cell apoptosis through the reactive oxygen species/apoptosis signal-regulating kinase 1 pathway in human osteosarcoma cells. Eur. J. Pharmacol 2008, 597, 19–26. [Google Scholar]
- Yang, B.; Reynolds, C.P. Tirapazamine cytotoxicity for neuroblastoma is p53 dependent. Clin. Cancer Res 2005, 11, 2774–2780. [Google Scholar]
- Huang, H.C.; Shi, G.Y.; Jiang, S.J.; Shi, C.S.; Wu, C.M.; Yang, H.Y.; Wu, H.L. Thrombomodulin-mediated cell adhesion: Involvement of its lectin-like domain. J. Biol. Chem 2003, 278, 46750–46759. [Google Scholar]
- Tseng, C.P.; Huang, C.L.; Huang, C.H.; Cheng, J.C.; Stern, A.; Tseng, C.H.; Chiu, D.T. Disabled-2 small interfering RNA modulates cellular adhesive function and MAPK activity during megakaryocytic differentiation of K562 cells. FEBS Lett 2003, 541, 21–27. [Google Scholar]
- Freedman, V.H.; Shin, S.I. Cellular tumorigenicity in nude mice: Correlation with cell growth in semi-solid medium. Cell 1974, 3, 355–359. [Google Scholar]
- Thornberry, N.A.; Rano, T.A.; Peterson, E.P.; Rasper, D.M.; Timkey, T.; Garcia-Calvo, M.; Houtzager, V.M.; Nordstrom, P.A.; Roy, S.; Vaillancourt, J.P.; et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem 1997, 272, 17907–17911. [Google Scholar]
- Nagata, S.; Suda, T. Fas and fas ligand: lpr and gld mutations. Immunol. Today 1995, 16, 39–43. [Google Scholar]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar]
- Fong, Y.C.; Yang, W.H.; Hsu, S.F.; Hsu, H.C.; Tseng, K.F.; Hsu, C.J.; Lee, C.Y.; Scully, S.P. 2-methoxyestradiol induces apoptosis and cell cycle arrest in human chondrosarcoma cells. J. Orthop. Res 2007, 25, 1106–1114. [Google Scholar]
- Arjmand, F.; Mohani, B.; Ahmad, S. Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu(II) complex. Eur. J. Med. Chem 2005, 40, 1103–1110. [Google Scholar]
- Wang, X.; Cheung, H.W.; Chun, A.C.; Jin, D.Y.; Wong, Y.C. Mitotic checkpoint defects in human cancers and their implications to chemotherapy. Front. Biosci 2008, 13, 2103–2114. [Google Scholar]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar]
- Susin, S.A.; Zamzami, N.; Castedo, M.; Daugas, E.; Wang, H.G.; Geley, S.; Fassy, F.; Reed, J.C.; Kroemer, G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J. Exp. Med 1997, 186, 25–37. [Google Scholar]
- Zamzami, N.; Brenner, C.; Marzo, I.; Susin, S.A.; Kroemer, G. Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene 1998, 16, 2265–2282. [Google Scholar]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar]
- Adams, J.M.; Cory, S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci 2001, 26, 61–66. [Google Scholar]
- Lockshin, R.A.; Zakeri, Z. Caspase-independent cell death? Oncogene 2004, 23, 2766–2773. [Google Scholar]
- Hsu, S.C.; Yang, J.S.; Kuo, C.L.; Lo, C.; Lin, J.P.; Hsia, T.C.; Lin, J.J.; Lai, K.C.; Kuo, H.M.; Huang, L.J.; et al. Novel quinolone CHM-1 induces apoptosis and inhibits metastasis in a human osterogenic sarcoma cell line. J. Orthop. Res 2009, 27, 1637–1644. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, J.-F.; Huang, Y.-L.; Yang, W.-H.; Chang, C.-S.; Tang, C.-H. 1-Benzyl-2-Phenylbenzimidazole (BPB), a Benzimidazole Derivative, Induces Cell Apoptosis in Human Chondrosarcoma through Intrinsic and Extrinsic Pathways. Int. J. Mol. Sci. 2012, 13, 16472-16488. https://doi.org/10.3390/ijms131216472
Liu J-F, Huang Y-L, Yang W-H, Chang C-S, Tang C-H. 1-Benzyl-2-Phenylbenzimidazole (BPB), a Benzimidazole Derivative, Induces Cell Apoptosis in Human Chondrosarcoma through Intrinsic and Extrinsic Pathways. International Journal of Molecular Sciences. 2012; 13(12):16472-16488. https://doi.org/10.3390/ijms131216472
Chicago/Turabian StyleLiu, Ju-Fang, Yuan-Li Huang, Wei-Hung Yang, Chih-Shiang Chang, and Chih-Hsin Tang. 2012. "1-Benzyl-2-Phenylbenzimidazole (BPB), a Benzimidazole Derivative, Induces Cell Apoptosis in Human Chondrosarcoma through Intrinsic and Extrinsic Pathways" International Journal of Molecular Sciences 13, no. 12: 16472-16488. https://doi.org/10.3390/ijms131216472




