Cinnamic Acid and Its Derivatives Inhibit Fructose-Mediated Protein Glycation
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of Cinnamic Acid and Its Derivatives on AGEs Formation
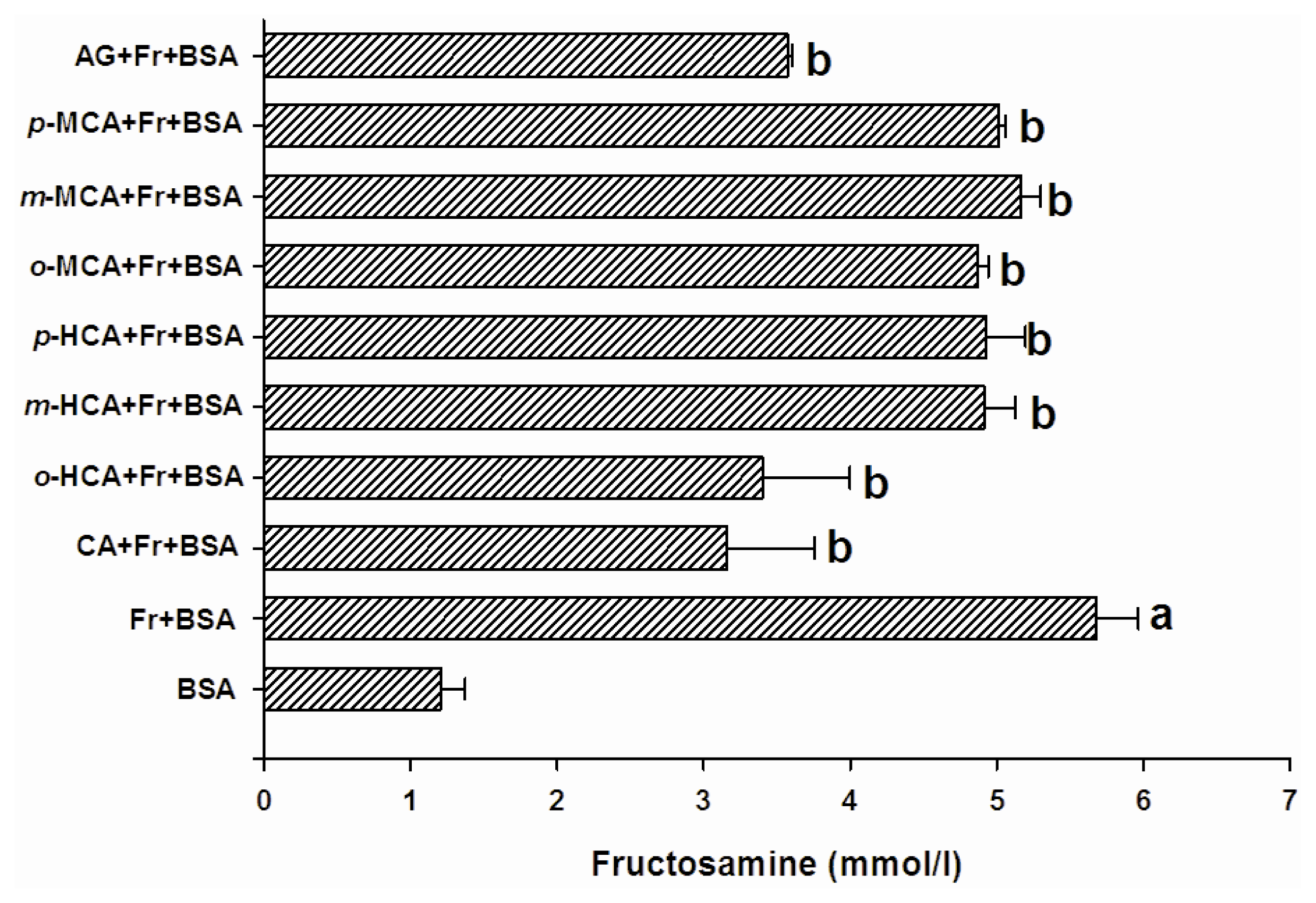
2.2. The Effect of Cinnamic Acid and its Derivatives on the Level of Fructosamine and the Formation of CML
2.3. The Effect of Cinnamic Acid and Its Derivatives on the Level of Amyloid Cross-β Structure
2.4. The Effect of Cinnamic Acid and its Derivatives on Glycation-Induced Protein Oxidation
3. Experimental Section
3.1. Chemicals
3.2. Glycation of Bovine Serum Albumin (BSA)
3.3. Determination of Fructosamine
3.4. Determination of Nɛ-(Carboxymethyl) Lysine (CML)
3.5. Determination of Amyloid Cross β Structure
3.6. Determination of Protein Carbonyl Content
3.7. Thiol Group Estimation
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Booth, A.A.; Khalifah, R.G.; Todd, P.; Hudson, B.G. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs). J. Biol. Chem 1997, 272, 5430–5437. [Google Scholar]
- Cohen, G.; Riahi, Y.; Alpert, E.; Gruzman, A.; Sasson, S. The roles of hyperglycaemia and oxidative stress in the rise and collapse of the natural protective mechanism against vascular endothelial cell dysfunction in diabetes. Arch. Physiol. Biochem 2007, 113, 259–267. [Google Scholar]
- Khazaei, M.R.; Bakhti, M.; Habibi-Rezaei, M. Nicotine reduces the cytotoxic effect of glycated proteins on microglial cells. Neurochem. Res 2010, 35, 548–558. [Google Scholar]
- Schmidt, A.M.; Stern, D. Atherosclerosis and diabetes: The RAGE connection. Curr. Atheroscler. Rep 2000, 2, 430–436. [Google Scholar]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med 1995, 146, 223–234. [Google Scholar]
- Wu, C.H.; Huang, S.M.; Lin, J.A.; Yen, G.C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct 2011, 2, 224–234. [Google Scholar]
- Bolton, W.K.; Cattran, D.C.; Williams, M.E.; Adler, S.G.; Appel, G.B.; Cartwright, K.; Foiles, P.G.; Freedman, B.I.; Raskin, P.; Ratner, R.E.; et al. ACTION I Investigator Group. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am. J. Nephrol 2004, 24, 32–40. [Google Scholar]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys 2003, 419, 31–40. [Google Scholar]
- Jin, S.; Cho, K.H. Water extracts of cinnamon and clove exhibits potent inhibition of protein glycation and anti-atherosclerotic activity in vitro and in vivo hypolipidemic activity in zebrafish. Food Chem. Toxicol 2011, 49, 1521–1529. [Google Scholar]
- Saraswat, M.; Reddy, P.Y.; Muthenna, P.; Reddy, G.B. Prevention of non-enzymic glycation of proteins by dietary agents: Prospects for alleviating diabetic complications. Br. J. Nutr 2009, 101, 1714–1721. [Google Scholar]
- Peng, X.; Ma, J.; Chao, J.; Sun, Z.; Chang, R.C.; Tse, I.; Li, E.T.; Chen, F.; Wang, M. Beneficial effects of cinnamon proanthocyanidins on the formation of specific advanced glycation endproducts and methylglyoxal-induced impairment on glucose consumption. J. Agric. Food Chem 2010, 58, 6692–6696. [Google Scholar]
- Dearlove, R.P.; Greenspan, P.; Hartle, D.K.; Swanson, R.B.; Hargrove, J.L. Inhibition of protein glycation by extracts of culinary herbs and spices. J. Med. Food 2008, 11, 275–281. [Google Scholar]
- Lee, E.J.; Kim, S.R.; Kim, J.; Kim, Y.C. Hepatoprotective phenylpropanoids from Scrophularia buergeriana roots against CCl4-induced toxicity: Action mechanism and structure-activity relationship. Planta Med 2002, 68, 407–411. [Google Scholar]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and cinnamic acid derivatives as antioxidants: Structure-activity relation. J. Agric. Food Chem 1999, 47, 1453–1459. [Google Scholar]
- Liu, I.M.; Hsu, F.L.; Chen, C.F.; Cheng, J.T. Antihyperglycemic action of isoferulic acid in streptozotocin-induced diabetic rats. Br. J. Pharmacol 2000, 129, 631–636. [Google Scholar]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal alpha-glucosidase. J. Enzyme Inhib. Med. Chem 2009, 24, 1194–1200. [Google Scholar]
- Adisakwattana, S.; Moonsan, P.; Yibchok-Anun, S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J. Agric. Food Chem 2008, 56, 7838–7844. [Google Scholar]
- Shaw, J.N.; Baynes, J.W.; Thorpe, S.R. N epsilon-(carboxymethyl) lysine (CML) as a biomarker of oxidative stress in long-lived tissue proteins. Methods Mol. Biol 2002, 186, 129–137. [Google Scholar]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract 2005, 67, 3–21. [Google Scholar]
- Qin, B.; Panickar, K.S.; Anderson, R.A. Cinnamon: Potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. J. Diabetes Sci. Technol 2010, 4, 685–693. [Google Scholar]
- Morozumi, S. Isolation, purification, and antibiotic activity of o-methoxycinnamaldehyde from cinnamon. Appl. Environ. Microb 1978, 36, 577–583. [Google Scholar]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem 2006, 75, 333–366. [Google Scholar]
- Uversky, V.N.; Fink, A.L. Conformational constraints for amyloid fibrillation: The importance of being unfolded. Biochim. Biophys. Acta 2004, 1698, 131–153. [Google Scholar]
- Wang, S.S.S.; Good, T.A. An overview of Alzheimer’s disease. J. Chin. Inst. Chem. Eng 2005, 36, 533–559. [Google Scholar]
- Ardestani, A.; Yazdanparast, R. Inhibitory effects of ethyl acetate extract of Teucrium polium on in vitro protein glycoxidation. Food Chem.Toxicol 2007, 45, 2402–2411. [Google Scholar]
- Bouma, B.; Kroon-Batenburg, L.M.; Wu, Y.P.; Brünjes, B.; Posthuma, G.; Kranenburg, O.; de Groot, P.G.; Voest, E.E.; Gebbink, M.F. Glycation induces formation of amyloid cross-beta structure in albumin. J. Biol. Chem 2003, 278, 41810–41819. [Google Scholar]
- Mossine, V.V.; Linetsky, M.; Glinsky, G.V.; Ortwerth, B.J.; Feather, M.S. Superoxide free radical generation by Amadori compounds: The role of acyclic forms and metal ions. Chem. Res. Toxicol 1999, 12, 230–236. [Google Scholar]
- Sharma, S.D.; Pandey, B.N.; Mishra, K.P.; Sivakami, S. Amadori product and age formation during nonenzymatic glycosylation of bovine serum albumin in vitro. J. Biochem. Mol. Biol. Biophys 2002, 6, 233–242. [Google Scholar]
- Johnson, R.N.; Metcalf, P.A.; Baker, J.R. Fructosamine: A new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin. Chim. Acta 1983, 127, 87–95. [Google Scholar]
- Le Vine, H., III. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999, 309, 274–284. [Google Scholar]
- Uchida, K.; Kanematsu, M.; Sakai, K.; Matsuda, T.; Hattori, N.; Mizuno, Y.; Suzuki, D.; Miyata, T.; Noguchi, N.; Niki, E.; et al. Protein-bound acrolein: Potential markers for oxidative stress (covalent modification of protein/antibody/atherosclerosis). Proc. Natl. Acad. Sci. USA 1998, 95, 4882–4887. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys 1959, 82, 70–77. [Google Scholar]





| Compound | Thiol group (nmol/mg protein) | Protein carbonyl content (nmol/mg protein) |
|---|---|---|
| BSA | 0.89 ± 0.01 | 0.25 ± 0.02 |
| BSA+Fr | 0.63 ± 0.03 a | 2.30 ± 0.08 a |
| BSA+Fr+ CA | 0.75 ± 0.06 b | 1.90 ± 0.07 b |
| BSA+Fr+ o-HCA | 0.71 ± 0.01 b | 1.88 ± 0.07 b |
| BSA+Fr+ m-HCA | 0.69 ± 0.04 b | 1.97 ± 0.05 b |
| BSA+Fr+ p-HCA | 0.80 ± 0.02 b | 1.84 ± 0.05 b |
| BSA+Fr+ o-MCA | 0.76 ± 0.02 b | 1.75 ± 0.07 b |
| BSA+Fr+ m-MCA | 0.76 ± 0.03 b | 1.84 ± 0.04 b |
| BSA+Fr+ p-MCA | 0.74 ± 0.01 b | 1.71 ± 0.10 b |
| BSA+Fr+AG | 0.81 ± 0.02 b | 1.59 ± 0.77 b |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Adisakwattana, S.; Sompong, W.; Meeprom, A.; Ngamukote, S.; Yibchok-anun, S. Cinnamic Acid and Its Derivatives Inhibit Fructose-Mediated Protein Glycation. Int. J. Mol. Sci. 2012, 13, 1778-1789. https://doi.org/10.3390/ijms13021778
Adisakwattana S, Sompong W, Meeprom A, Ngamukote S, Yibchok-anun S. Cinnamic Acid and Its Derivatives Inhibit Fructose-Mediated Protein Glycation. International Journal of Molecular Sciences. 2012; 13(2):1778-1789. https://doi.org/10.3390/ijms13021778
Chicago/Turabian StyleAdisakwattana, Sirichai, Weerachat Sompong, Aramsri Meeprom, Sathaporn Ngamukote, and Sirintorn Yibchok-anun. 2012. "Cinnamic Acid and Its Derivatives Inhibit Fructose-Mediated Protein Glycation" International Journal of Molecular Sciences 13, no. 2: 1778-1789. https://doi.org/10.3390/ijms13021778






