Dietary Pseudopurpurin Effects on Bone Mineral Density and Bone Geometry Architecture in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. ESI-MS Analyses
2.2. Body Weight and Femur Length in Rats
2.3. Bone Mineral Composition and Bone Mineral Density
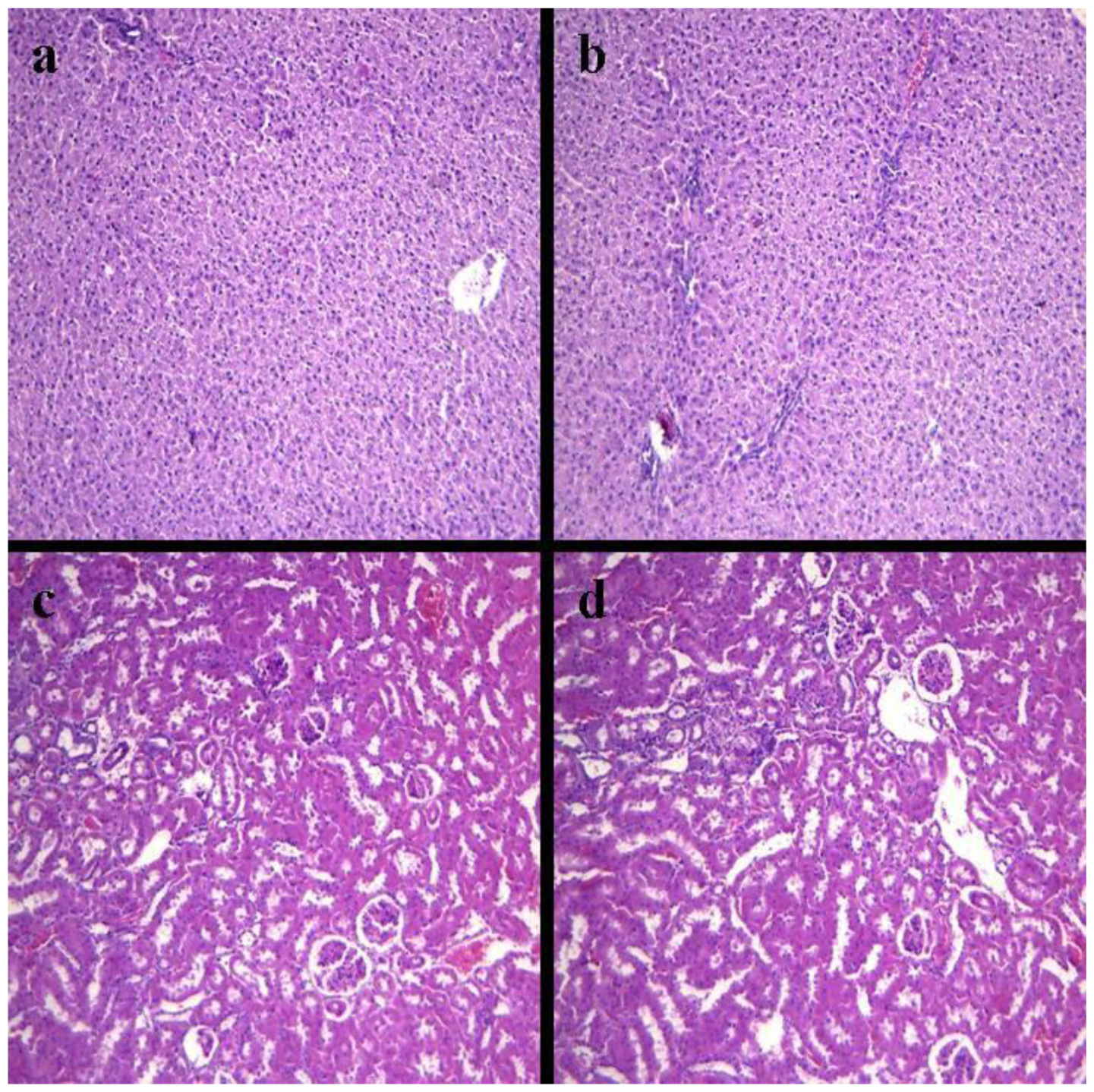
2.4. Histopathology of Internal Organs
2.5. Biochemical Evaluations
2.6. Femur Micro-CT
2.7. Affinity of Pseudopurpurin and Calcium
2.8. Discussion
3. Materials and Methods
3.1. Preparation of Pseudopurpurin
3.2. Semi-Preparative HPLC
3.3. Electrospray Ionization-Mass Spectrometry (ESI-MS)
3.4. Animals
3.5. Bone Mineral Elements
3.6. Dual Energy X-ray Absorptiometry
3.7. Histopathological Analysis
3.8. Detection of Blood of Rats
3.9. Micro-CT Scanning
3.10. Affinity of Pseudopurpurin and Calcium Test
3.11. Statistical Analyses
4. Conclusions
Acknowledgements
References
- Duhamel, H. Sur une racine qui a la faculte de teindre en rouge les os des animaux vivants. Mem. Acad. Roy. Sci 1739, 79, 1–13. [Google Scholar]
- Hunter, J. Experiments and observations on the growth of bones. Bone 1798, 7, 315–330. [Google Scholar]
- Moore, A.W. Head growth of the macaque monkey as revealed by vital staining, embedding and undecalcified sectioning. Am. J. Orthod 1949, 35, 654–671. [Google Scholar]
- Massler, M.; Schour, I. The growth pattern of the cranial vault in the albino rat as measured by vital staining with alizarine red S. Anat. Rec 1951, 110, 83–101. [Google Scholar]
- Baer, M.J. Pattern of growth of the skull as revealed by vital staining. Hum. Biol 1954, 26, 80–126. [Google Scholar]
- Hoyte, D.A.N. The postnatal growth of the ear capsule in the rabbit. Am. J. Anat 1961, 108, 1–16. [Google Scholar]
- Vilmann, H. The growth of the parietal bone in the albino rat studied by roentgenocephalometry and vital staining. Arch. Oral Biol 1968, 13, 887–902. [Google Scholar]
- Derksen, G.C.H.; van Beek, T.A. Studies in Natural Products Chemistry; Elsevier: Amsterdam, the Netherlands, 2002; Volume 6, p. 629. [Google Scholar]
- Nakanishi, F.; Nagasawa, Y.; Kabaya, Y.; Sekimoto, H. Characterization of lucidin formation in Rubia tinctorum L. Plant Phys. Biochem 2005, 43, 921–928. [Google Scholar]
- Paff, G.H.; Eksterowics, F.C. The selective stoppage of bone growth in tissue culture. Anat. Rec 1950, 108, 45–55. [Google Scholar]
- Schour, I. Measurements of bone growth by alizarine injections. Proc. Soc. Exp. Biol. Med 1936, 34, 140–141. [Google Scholar]
- Hoyte, D.A.N. Alizarin as an indicator of bone growth. J. Anat 1960, 94, 432–442. [Google Scholar]
- Derksen, G.C.; Niederländer, H.A.; van Beek, T.A. Analysis of anthraquinones in Rubia tinctorum L. by liquid chromatography coupled with diode-array UV and mass spectrometric detection. J. Chromatogr. A 2002, 978, 119–127. [Google Scholar]
- Richter, D. Vital staining of bones with madder. Biochem. J 1937, 31, 591–595. [Google Scholar]
- Schweppe, H. Handbuch der Naturfarbstoffe: Vorkommen, Verwendung, Nachweis; Ecomed Verlagsgesellschaft: Landsberg, Germany, 1992. [Google Scholar]
- Nigel, K. Genetics of calcium-sensing-regulation of calcium levels in the body. Curr. Opin. Pharmacol 2003, 3, 291–294. [Google Scholar]
- Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red S stains for calcium. J. Histochem. Cytochem 1969, 17, 110–124. [Google Scholar]
- Kiel, E.G.; Heertjes, P.M. Metal complexes of alizarin I—The structure of the calcium-aluminium lake of alizarin. J. Soc. Dyers Colour 1963, 79, 21–27. [Google Scholar]
- Kiel, E.G.; Heertjes, P.M. Metal complexes of alizarin II—The structure of some metal complexes of alizarin other than Turkey red. J. Soc. Dyers Colour 1963, 79, 61–64. [Google Scholar]
- Kiel, E.G.; Heertjes, P.M. Metal complexes of alizarin III—The structure of metal complexes of some 3-derivatives of alizarin. J. Soc. Dyers Colour 1963, 79, 186–188. [Google Scholar]
- Kiel, E.G.; Heertjes, P.M. Metal complexes of alizarin IV—The structure of the potassium and calcium salts of alizarin and of 3-nitroalizarin. J. Soc. Dyers Colour 1963, 79, 363–367. [Google Scholar]
- Sudo, H.; Kodama, H.A.; Amagai, Y. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J. Cell Biol 1983, 96, 191–198. [Google Scholar]
- Fratzl-Zelman, N.; Fratzl, P.; Horandner, H. Matrix mineralization in MC3T3-E1 cell cultures initiated by beta-glycerophosphate pulse. Bone 1998, 23, 511–520. [Google Scholar]
- Nordin, B.E. Calcium and osteoporosis. Nutrition 1997, 13, 664–686. [Google Scholar]
- Wolf, F.I.; Cittadini, A. Chemistry and biochemistry of magnesium. Mol. Aspects Med 2003, 24, 3–9. [Google Scholar]
- Mongiorgi, R.; Gnudi, S.; Moroni, A.; Bertocchi, G.; Galliani, I.; Benfenati, L. Bone mineral alterations and Mg content in aging. Boll. Soc. Ital. Biol. Sper 1990, 66, 623–630. [Google Scholar]
- Saltman, P.D.; Strause, L.G. The role of trace minerals in osteoporosis. J. Am. Coll. Nutr 1993, 12, 384–389. [Google Scholar]
- Barker, D.J.P.; Chamberlain, A.T.; Detheridge, F.M.; Gardner, M.J. Low lead levels in pagetoid bone. Metab. Bone Dis. Relat. Res 1982, 4, 243–247. [Google Scholar]
- Murray, E.J.; Messer, H.H. Turnover of zinc during normal and accelerated bone loss in rats. J. Nutr 1981, 111, 1641–1647. [Google Scholar]
- Rabinowitz, M.B. Toxokinetics of bone lead. Environ. Health Persp 1991, 91, 33–37. [Google Scholar]
- Wittmers, L.E.; Wallgren, J.; Alich, A. Lead in bone. IV. Distribution of lead in the human skeleton. Arch. Environ. Health 1988, 43, 381–390. [Google Scholar]
- Helliwell, T.R.; Kelly, S.A.; Walsh, H.P.; Klenerman, L.; Haines, J.; Clark, R.; Roberts, N.B. Elemental analysis of femoral bone from patients with fractured neck of femur or osteoarthrosis. Bone 1996, 18, 151–157. [Google Scholar]
- Sadaaki, T.; Yoshitaka, Y.; Yoichi, S.; Yoshiaki, D.; Tomokazu, H. Low calcium environment effects osteoprotegerin ligand/osteoclast differentiation factor. Biochem. Biophys. Res. Commun 2000, 276, 524–529. [Google Scholar]
- Yoneda, N.; Saito, S.; Kimura, M.; Yamada, M.; Iida, M.; Murakami, T.; Irahara, M.; Shima, K.; Aono, T. The influence of ovariectomy on ob gene expression in rats. Horm. Metab. Res 1998, 30, 263–265. [Google Scholar]
- Kimura, M.; Irahara, M.; Yasui, T.; Saito, S.; Tezuka, M.; Yamano, S.; Kamada, M.; Aono, T. The obesity in bilateral ovariectomized rats is related to a decrease in the expression of leptin receptors in the brain. Biochem. Biophys. Res. Commun 2002, 290, 1349–1353. [Google Scholar]
- Kurasawa, K. Bone quality and biochemical markers. Clin. Calcium 2005, 15, 959–964. [Google Scholar]
- Kazakia, G.J.; Majumdar, S. New imaging technologies in the diagnosis of osteoporosis. Rev. Endocr. Metab. Disord 2006, 7, 67–74. [Google Scholar]
- Teo, J.C.; Si-Hoe, K.M.; Keh, J.E.; Teoh, S.H. Relationship between CT intensity, micro-architecture and mechanical properties of porcine vertebral cancellous bone. Clin. Biomech 2006, 21, 235–244. [Google Scholar]
- Lane, N.E.; Kumer, J.L.; Majumdar, S.; Khan, M.; Lotz, J.; Stevens, R.E.; Klein, R.; Phelps, K.V. The effects of synthetic conjugated estrogens, a (cenestin) on trabecular bone structure and strength in the ovariectomized rat model. Osteoporos. Int 2002, 13, 816–823. [Google Scholar]
- Bergh, J.J.; Xu, Y.; Farach-Carson, M.C. Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel. Endocrinology 2000, 145, 426–436. [Google Scholar]




| Body weight (g) | Femur length (cm) | ||
|---|---|---|---|
| 0.5% group | 0.5 month | 24.5 ± 5.66 | 0.81 ± 0.057 |
| 1 month | 57.3 ± 9.37 | 1.78 ± 0.72 | |
| 2 months | 123.6 ± 22.2 | 2.12 ± 0.92 | |
| 0% group | 0.5 month | 23.8 ± 6.01 | 0.79 ± 0.034 |
| 1 month | 54.6 ± 11.57 | 1.55 ± 0.83 | |
| 2 months | 116 ± 21.5 | 1.94 ± 0.94 |
| AST (U/L) | ALT (U/L) | BUN (mmol/L) | CRE (umol/L) | |
|---|---|---|---|---|
| 0.5% group | 82.40 ± 5.47 | 31.24 ± 3.17 | 11.52 ± 1.44 | 22.38 ± 1.44 |
| 0% group | 83.24 ± 5.99 | 31.45 ± 2.66 | 10.79 ± 1.24 | 21.02 ± 1.54 |
| BV/TV (%) | Tb.Th (mm) | Tb.N (/mm) | Tb.Sp (um) | SMI | ||
|---|---|---|---|---|---|---|
| 0% group rats | 0.5 month | 2.32 ± 0. 3 | 0.051 ± 0.009 | 2.76 ± 0.58 | 0.365 ± 0.03 | 2.22 ± 0.3 |
| 0.5% group rats | 2.38 ± 0.5 | 0.054 ± 0.01 | 2.77 ± 0.69 | 0.370 ± 0.037 | 2.20 ± 0.4 | |
| 0% group rats | 1 month | 3.16 ± 0.04 | 0.08 ± 0.007 | 3.16 ± 1.11 | 0.295 ± 0.013 | 1.92 ± 0.03 |
| 0.5% group rats | 3.82 ± 0.03 * | 0.13 ± 0.007 * | 4.01 ± 1.07 * | 0.216 ± 0.014 * | 1.30 ± 0.02 * | |
| 0% group rats | 2 months | 5.61 ± 0.08 | 0.15 ± 0.05 | 3.99 ± 1.0 | 0.218 ± 0.003 | 1.41 ± 0.011 |
| 0.5% group rats | 6.75 ± 0.08 * | 0.23 ± 0.04 * | 5.16 ± 1.3 * | 0.167 ± 0.005 * | 0.86 ± 0.01 * |
| Mean Thickness (mm) | Inner Perimeter (mm) | Outer Perimeter (mm) | Marrow Area (mm2) | Cortical Area (mm2) | Total Area (mm2) | ||
|---|---|---|---|---|---|---|---|
| 0% group rats | 0.5 month | 0.17 ± 0.03 | 4.21 ± 0.9 | 5.7 ± 1.1 | 2.76 ± 0.08 | 0.71 ± 0.03 | 3.22 ± 0.7 |
| 0.5% group rats | 0.18 ± 0.05 | 4.42 ± 1.0 | 6.34 ± 1.1 | 2.77 ± 0.09 | 0.718 ± 0.037 | 3.20 ± 0.8 | |
| 0% group rats | 1 month | 0.241 ± 0.05 | 6.57 ± 1.0 | 8.28 ± 1.54 | 3.34 ± 0.67 | 1.86 ± 0.36 | 5.33 ± 1.64 |
| 0.5% group rats | 0.267 ± 0.03 | 6.82 ± 0.88 | 8.41 ± 1.33 | 3.54 ± 0.78 | 1.99 ± 0.34 | 5.35 ± 1.55 | |
| 0% group rats | 2 months | 0.38 ± 0.09 | 7.56 ± 1.82 | 10.87 ± 3.1 | 4.01 ± 1.64 | 3.75 ± 1.22 | 7.16 ± 2.31 |
| 0.5% group rats | 0.43 ± 0.07 * | 8.41 ± 1.34 * | 13.88 ± 2.87 * | 5.17 ± 1.77 * | 4.8 ± 1.12 * | 8.13 ± 2.13 * |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, C.-C.; Li, X.-B.; Han, T.-S.; Li, P.; Liu, G.-W.; Wang, W.-Z.; Wang, Z. Dietary Pseudopurpurin Effects on Bone Mineral Density and Bone Geometry Architecture in Rats. Int. J. Mol. Sci. 2012, 13, 3431-3443. https://doi.org/10.3390/ijms13033431
Wu C-C, Li X-B, Han T-S, Li P, Liu G-W, Wang W-Z, Wang Z. Dietary Pseudopurpurin Effects on Bone Mineral Density and Bone Geometry Architecture in Rats. International Journal of Molecular Sciences. 2012; 13(3):3431-3443. https://doi.org/10.3390/ijms13033431
Chicago/Turabian StyleWu, Chen-Chen, Xiao-Bing Li, Tie-Suo Han, Peng Li, Guo-Wen Liu, Wei-Zhong Wang, and Zhe Wang. 2012. "Dietary Pseudopurpurin Effects on Bone Mineral Density and Bone Geometry Architecture in Rats" International Journal of Molecular Sciences 13, no. 3: 3431-3443. https://doi.org/10.3390/ijms13033431
APA StyleWu, C.-C., Li, X.-B., Han, T.-S., Li, P., Liu, G.-W., Wang, W.-Z., & Wang, Z. (2012). Dietary Pseudopurpurin Effects on Bone Mineral Density and Bone Geometry Architecture in Rats. International Journal of Molecular Sciences, 13(3), 3431-3443. https://doi.org/10.3390/ijms13033431





