Effect of Polyethylene Glycol Modification of TiO2 Nanoparticles on Cytotoxicity and Gene Expressions in Human Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
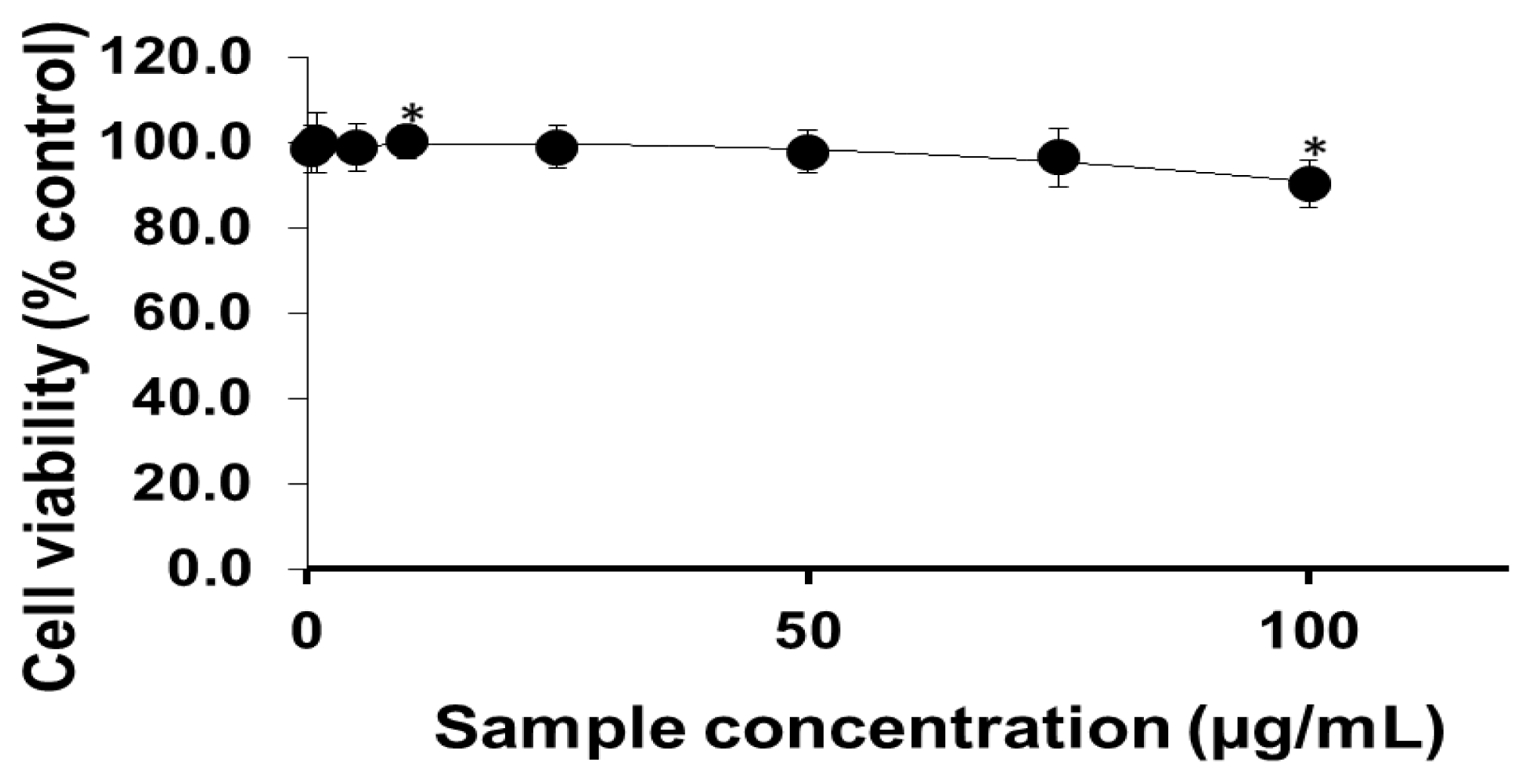
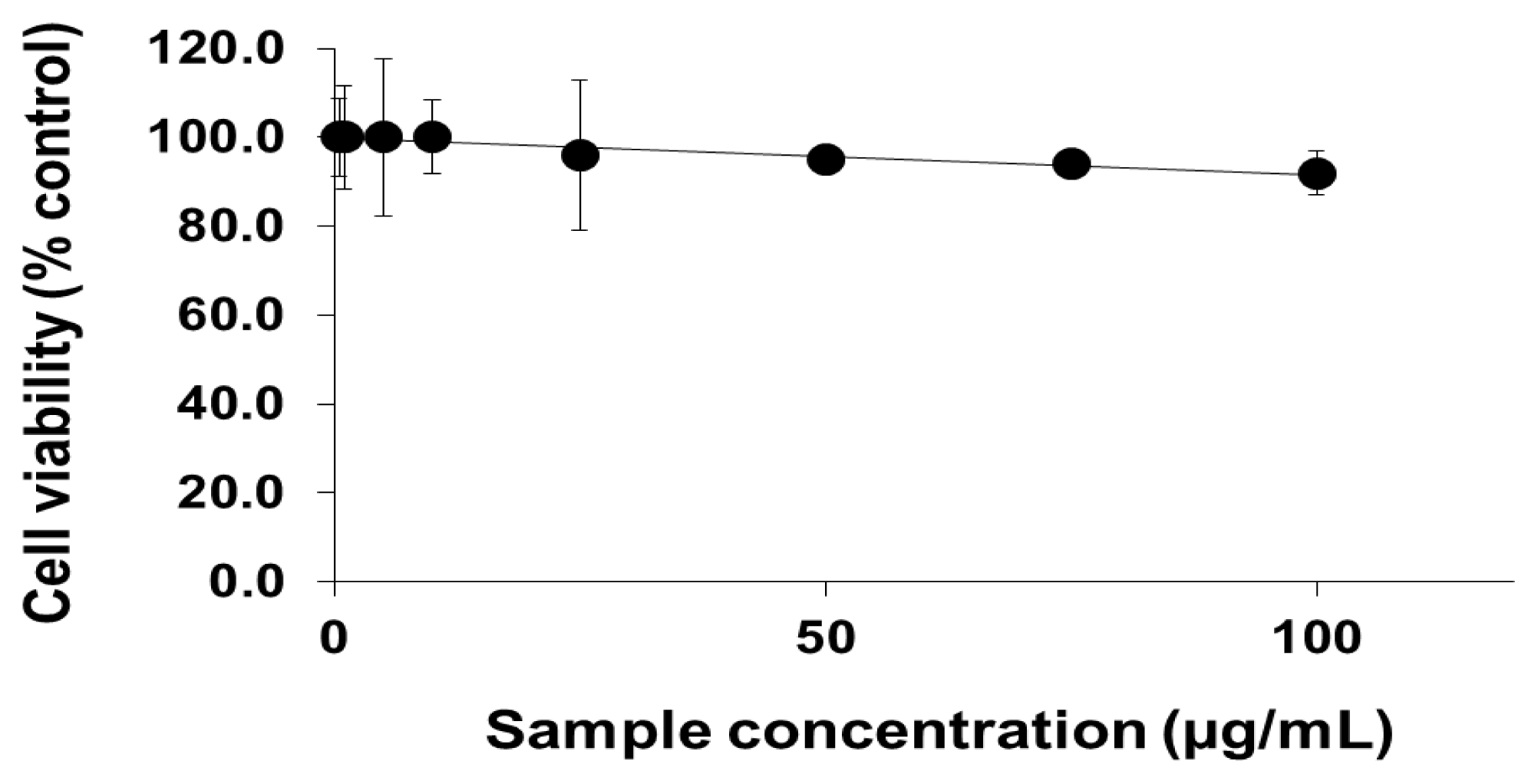
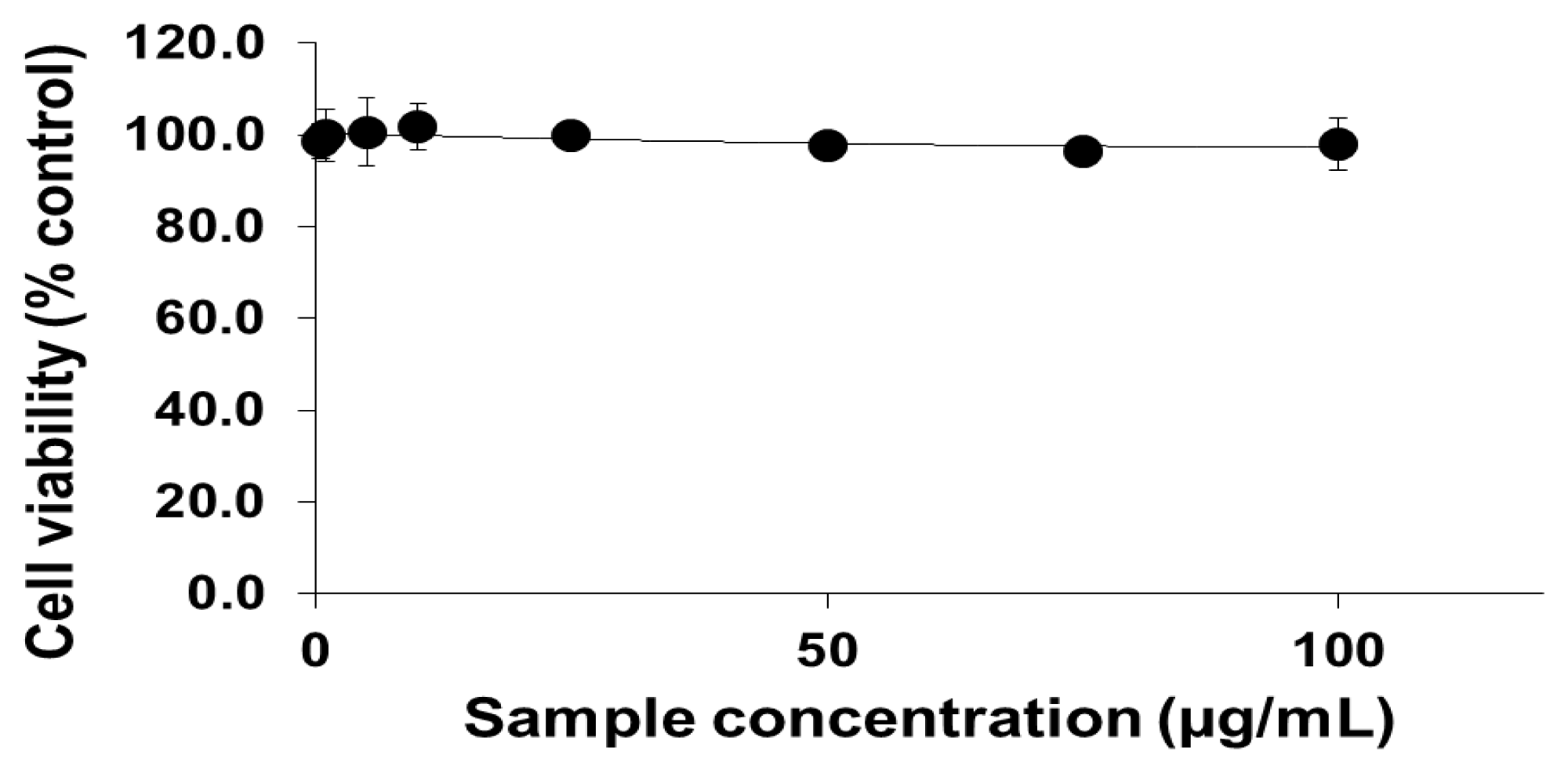
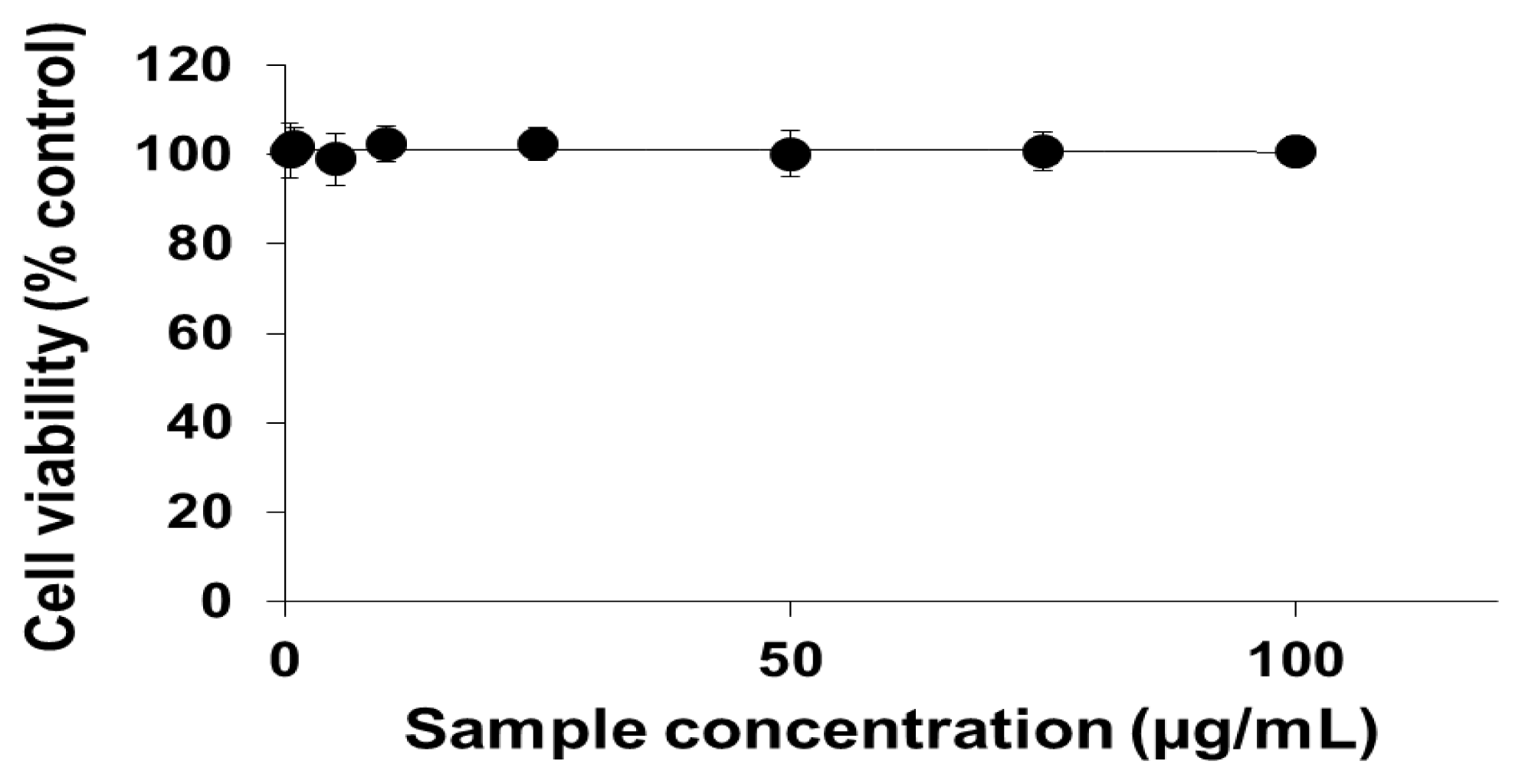
2.1. Viability of Cells Exposed to PEG-TiO2 NPs
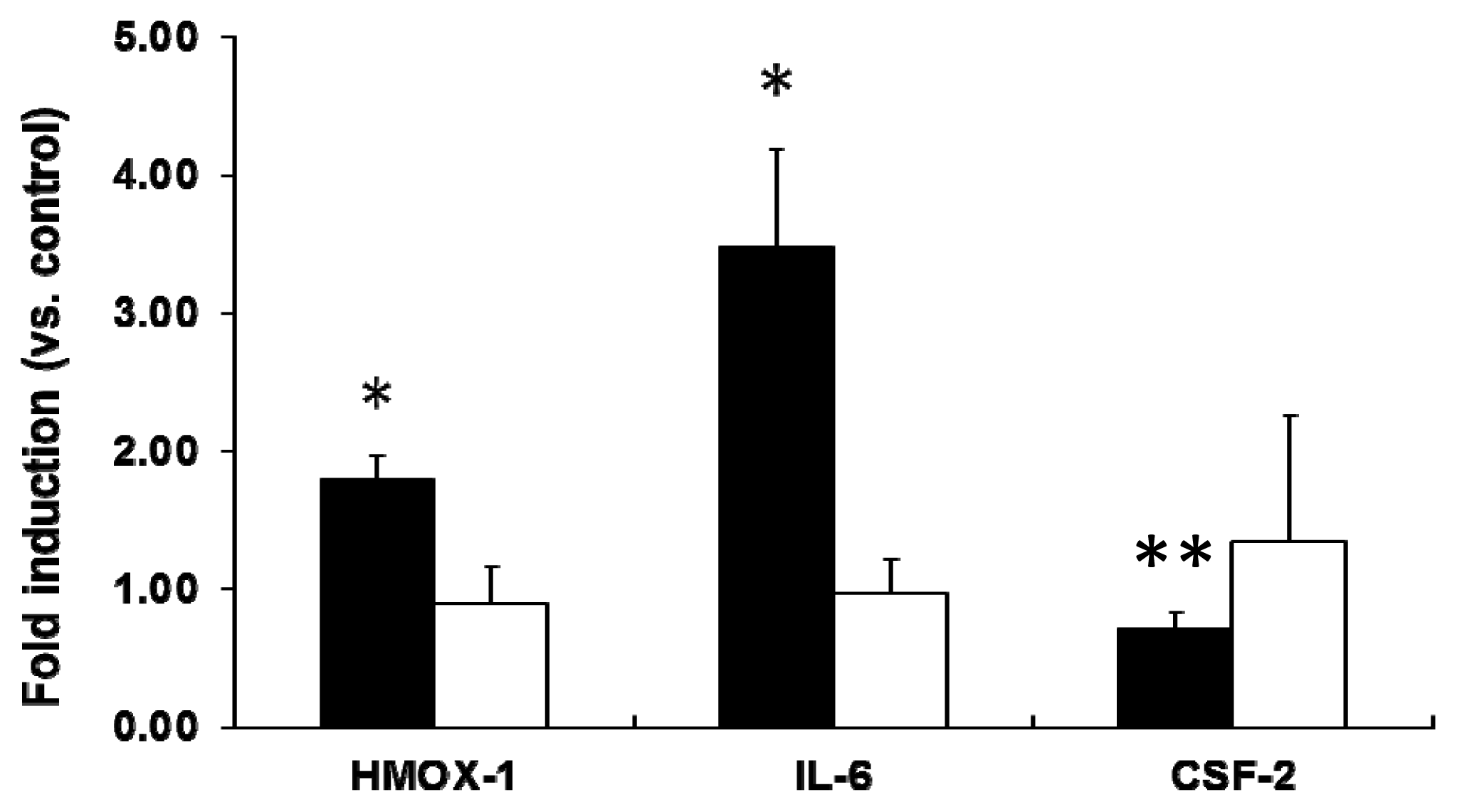
2.2. mRNA Expression Analysis of NP-Exposed Cells
2.3. Discussion
3. Experimental Section
3.1. Preparation of PEG-TiO2
3.2. Cell Culture
3.3. Cell Viability Test
3.4. Gene Expression Analysis
3.4.1. PCR Array
3.4.2. Real-Time (RT) PCR
3.5. Statistical Analysis
4. Conclusions
References
- Ng, C.T.; Li, J.J.; Perumalsamy, R.; Watt, F.; Yung, L.Y.L.; Bay, B.H. Localizing cellular uptake of nanomaterials in vitro by transmission electron microscopy. Microsc. Sci. Technol. Appl. Educ 2010, 1, 316–320. [Google Scholar]
- Ratyakshi; Chauhan, R.P. Colloidal synthesis of silver nanoparticles. Asian J. Chem 2009, 21, S113–S116. [Google Scholar]
- Huang, C.C.; Aronstam, R.S.; Chen, D.R.; Huang, Y.W. Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol. In Vitro 2010, 24, 45–55. [Google Scholar]
- Pujalte, I.; Passagne, I.; Brouillaud, B.; Treguer, M.; Durand, E.; Courtes, C.O.; Lazou, B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol 2011, 8. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modification and applications. Chem. Rev 2007, 107, 2891–2959. [Google Scholar]
- Chen, E.; Ruvalcaba, M.; Araujo, L.; Chapman, R.; Chin, W.C. Ultrafine titanium dioxide nanoparticles induce cell death in human bronchial epithelial cells. J. Exp. Nanosci 2008, 3, 171–183. [Google Scholar]
- Gurr, J.R.; Wang, A.S.S.; Chen, C.H.; Jan, K.Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar]
- Yamaguchi, S.; Kobayashi, H.; Narita, T.; Kanehira, K.; Sonezaki, S.; Kudo, N.; Kubota, Y.; Terasaka, S.; Houkin, K. Sonodynamic therapy using water-dispersed TiO2-polyethylene glycol compound on glioma cells: Comparison of cytotoxic mechanism with photodynamic therapy. Ultrason. Sonochem 2011, 18, 1197–1204. [Google Scholar]
- Oberdorster, G.; Ferin, J.; Gelein, R.; Soderholm, S.C.; Finkelstein, J. Role of alveolar macrophages in lung injury studies with ultra fine particles. Environ. Health Perspect 1992, 97, 193–199. [Google Scholar]
- Baggs, R.B.; Fern, J.; Oberdorster, G. Regression of pulmonary lesions produced by inhaled titanium dioxide in rats. Vet. Pathol 1997, 34, 592–597. [Google Scholar]
- Mishra, V.K.; Mishra, S.K.; Jha, A. Application of nanomaterials in mesenchymal stem cell engineering. Dig. J. Nanomater. Biostructures 2008, 3, 203–208. [Google Scholar]
- Jokerst, J.V.; Lobovkin, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar]
- Wang, W.; Xiong, W.; Wan, J.; Sun, X.; Xu, H.; Yang, X. The decrease of PAMAM dendrimer-induced cytotoxicity by PEGylation via attenuation of oxidative stress. Nanotechnology 2009, 20, 105103–105109. [Google Scholar]
- Matsumura, S.; Sato, S.; Yadasaka, M.; Tomida, A.; Tsuruo, T.; Iijima, S.; Shiba, K. Prevention of carbon nanohorn agglomeration using a conjugate composed of comb-shaped polyethylene glycol and a peptide aptamer. Mol. Pharmaceutics 2009, 6, 441–447. [Google Scholar]
- Eck, W.; Craig, G.; Sigdel, A.; Ritter, G.; Old, L.J.; Tang, L.; Brennan, M.F.; Allen, P.J.; Mason, M.D. PEGylated gold nanoparticles conjugated to monoclonal F19 antibodies as targeted labeling agents for human pancreatic carcinoma tissue. ACS Nano 2008, 2, 2263–2272. [Google Scholar]
- Prencipe, G.; Tabakman, S.M.; Welsher, K.; Liu, Z.; Goodwin, A.P.; Zhang, L.; Henry, J.; Dai, H. PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J. Am. Chem. Soc 2009, 131, 4783–4787. [Google Scholar]
- Zhang, Y.; Xu, Y.; Li, Z.; Chen, T.; Lantz, S.M.; Howard, P.C.; Paule, M.G.; Slikker, W., Jr; Watanabe, F.; Mustafa, T.; et al. PEGylated single-walled carbon nanotubes in neuronal PC12 cells. ACS Nano 2011, 5, 7020–7033. [Google Scholar]
- Park, E.J.; Yoon, J.; Choi, K.; Li, J.; Park, K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology 2009, 260, 37–46. [Google Scholar]
- Shimazaki, J.O.; Takaku, S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Effects of titanium dioxide nanoparticle aggregate size on gene expression. Int. J. Mol. Sci 2010, 11, 2383–2392. [Google Scholar]
- Chen, P.; Migita, S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Development of sensor cells using NF-κB pathway activation for detection of nanoparticle induced inflammation. Sensors 2011, 11, 7219–7230. [Google Scholar]
- Sharma, V.K. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment. J. Environ. Sci. Health Part A: Toxic/Hazard. Subst. Environ. Eng 2009, 44, 1485–1495. [Google Scholar]
- Hamilton, R.F.; Wu, N.; Porter, D.; Buford, M.; Wolfarth, M.; Holian, A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part. Fibre Toxicol 2009, 6. [Google Scholar] [CrossRef]
- Jin, C.Y.; Zhu, B.S.; Wang, X.F.; Lu, Q.H. Cytotoxicity of TiO2 nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol 2008, 21, 1871–1877. [Google Scholar]
- Pan, W.; Lee, L.; Slutsky, R.; Clak, A.F.; Pernodet, N.; Rafailovich, M.H. Adverse effects of TiO2 nanoparticles on human dermal fibroblasts and how to protect cells. Small 2009, 4, 511–520. [Google Scholar]
- Long, T.C.; Tajuba, J.; Sama, P.; Saleh, N.; Swartz, C.; Parker, J.; Hester, S.; Lowry, G.V.; Veronesi, B. Nanosize TiO2 stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ. Health Perspect 2007, 115, 1631–1637. [Google Scholar]
- Warheit, D.B.; Webb, T.R.; Sayes, C.M.; Colvin, V.L.; Reed, K.L. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area. Toxicol. Sci 2006, 91, 227–236. [Google Scholar]
- Shimizu, M.; Tainaka, H.; Oba, T.; Mizuo, K.; Umezawa, M.; Takeda, K. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part. Fibre Toxicol 2009, 6. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, J.; Tong, Y.W.; Wang, C.H. Effects of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the Hep G2 cells. J. Controlled Release 2007, 118, 7–17. [Google Scholar]
- Tangaraja, A.; Savitha, V.; Jegatheesan, K. Preparation and characterization of polyethylene glycol coated silica nanoparticles for drug delivery application. Comp. Gen. Pharmacol 2010, 4, 31–38. [Google Scholar]
- Molavi, B.; Mehta, J.L. Oxidative stress in cardiovascular disease: Molecular basis of its deleterious effects, its detection, and therapecutic considerations. Curr. Opin. Cardiol 2004, 19, 488–493. [Google Scholar]
- Mann, D.L. Stress activated cytokines and the heart: From adaptation to maladaptation. Annu. Rev. Physiol 2003, 65, 81–101. [Google Scholar]
- Oberdorster, G.; Ferin, J.; Lehnert, B.E. Correlation between particle size, in vivo particle persistence and lung injury. Environ. Health Perspect 1994, 102, 173–179. [Google Scholar]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 3, 4930–4934. [Google Scholar]
- Garnett, M.C.; Kallinteri, P. Nanomedicines and nanotoxicology: Some physiological principles. Occup. Med 2006, 56, 307–311. [Google Scholar]
- Anderem, A.; Underhill, D.M. Mechanism of phagocytosis in macrophages. Annu. Rev. Immunol 1999, 17, 593–623. [Google Scholar]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar]
- Zhang, L.W.; Riviere, N.A.M. Mechanism of quantum dot nanoparticle cellular uptake. Toxicol. Sci 2009, 110, 138–155. [Google Scholar]
- Hansen, C.G.; Nichols, B.J. Molecular mechanisms of clathrin-independent endocytosis. J. Cell Sci 2009, 122, 1713–1721. [Google Scholar]
- Xiao, Y.; Forry, S.P.; Gao, X.; Holbrook, R.D.; Telford, W.D.; Tona, A. Dynamics and mechanisms of quantum dot nanoparticle cellular uptake. J. Nanobiotechnol 2010, 8. [Google Scholar] [CrossRef]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol 2007, 8, 603–612. [Google Scholar]
- Ishibashi, Y.; Imai, S.; Inouye, Y.; Okano, T.; Taniguchi, A. Effects of carbocisteine on sialyl-Lewis x expression in NCI-H292 cells, an airway carcinoma cell line, stimulated with tumor necrosis factor-α. Eur. J. Pharmacol 2006, 530, 223–228. [Google Scholar]
- Nudejima, S.; Miyazawa, K.; Shimazaki, J.O.; Taniguchi, A. Observation of phagocytosis of fullerene nanowhiskers by PMA-treated THP-1 cells. J. Phys. Conf. Ser 2009, 159, 1–6. [Google Scholar]
- Shimazaki, J.O.; Nudejima, S.; Takaku, S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Effects of fullerene nanowhiskers on cytotoxicity and gene expression. Health 2010, 2, 1463–1466. [Google Scholar]
- Zheng, Y.; Li, R. In vitro and in vivo biocompatibility studies on ZnO nanoparticles. Int. J. Mod. Phys. B 2009, 23, 1566–1571. [Google Scholar]
- Piret, J.P.; Vankoningsloo, S.; Noel, F.; Mendoza, J.M.; Lucas, S.; Saout, C.; Toussaint, O. Inflammation response at the transcriptional level of HepG2 cells induced by multi-walled carbon nanotubes. J. Phys. Conf. Ser 2011, 304. [Google Scholar] [CrossRef]
- Kawata, K.; Osawa, M.; Okabe, S. In vitro toxicity of silver nanoparticles at nanocytotoxic doses to Hep G2 human hepatoma cells. Environ. Sci. Technol 2009, 43, 6046–6051. [Google Scholar]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar]
- Matsumoto, A.; Harada, H.; Saito, M.; Taniguchi, A. Induction of enamel matrix protein expression in an ameloblast cell line co-cultures with a mesenchymal cell line in vitro. In Vitro Cell. Dev. Biol. Anim 2011, 47, 39–44. [Google Scholar]



| Symbols of Genes | Description of the Genes |
|---|---|
| CCNG1 | Cyclin G1 |
| CRYAB | Crystallin, alpha B |
| CSF2 | Colony stimulating factor 2 (granulocyte- macrophage) |
| CYP2E1 | Cytochrome P450, family 2, subfamily E, polypeptide 1 |
| CYP7A1 | Cytochrome P450, family 7, subfamily A, polypeptide 1 |
| FMO1 | Flavin containing monooxygenase 1 |
| FMO5 | Flavin containing monooxygenase 5 |
| GSTM3 | Glutathione transferase M3 |
| HMOX1 | Heme oxygenase 1 |
| HSPA6 | Heat Shock 70kDa protein 6 (HSP70B’) |
| IL6 | Interleukin 6 (interferon, beta 2) |
| LTA | Lymphotoxin alpha (TNF superfamily, member 1) |
| TNF | Tumor necrosis factor (TNF superfamily, member 2) |
| UGT1A4 | UDP glucuronosyltransferase 1 family, polypeptide A4 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mano, S.S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Effect of Polyethylene Glycol Modification of TiO2 Nanoparticles on Cytotoxicity and Gene Expressions in Human Cell Lines. Int. J. Mol. Sci. 2012, 13, 3703-3717. https://doi.org/10.3390/ijms13033703
Mano SS, Kanehira K, Sonezaki S, Taniguchi A. Effect of Polyethylene Glycol Modification of TiO2 Nanoparticles on Cytotoxicity and Gene Expressions in Human Cell Lines. International Journal of Molecular Sciences. 2012; 13(3):3703-3717. https://doi.org/10.3390/ijms13033703
Chicago/Turabian StyleMano, Sharmy Saimon, Koki Kanehira, Shuji Sonezaki, and Akiyoshi Taniguchi. 2012. "Effect of Polyethylene Glycol Modification of TiO2 Nanoparticles on Cytotoxicity and Gene Expressions in Human Cell Lines" International Journal of Molecular Sciences 13, no. 3: 3703-3717. https://doi.org/10.3390/ijms13033703





