Expression of Sox2 and Oct4 and Their Clinical Significance in Human Non-Small-Cell Lung Cancer
Abstract
:1. Introduction
2. Results
2.1. Sox2 and Oct4 Expressions in Cancerous Tissues, Precancerous Tissues and Lung Benign Tumor Tissue
2.2. Clinicopathological Correlations
2.3. Correlation between Sox2 and Oct4 Expression
2.4. Relation to Survival
3. Discussion
4. Experimental Section
4.1. Specimens and Clinical Pathological Data
4.2. Imunohistochemical Analysis
4.3. RT-PCR
4.4. Western Blot Analysis
4.5. Follow-Up
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J Clin 2010, 60, 277–300. [Google Scholar]
- Leget, G.A.; Czuczman, M.S. Use of rituximab, the new FDA-approved antibody. Curr. Opin. Oncol 1998, 10, 548–551. [Google Scholar]
- Marrero, J.A.; Lok, A.S. Newer markers for hepatocellular carcinoma. Gastroenterology 2004, 127, S113–S119. [Google Scholar]
- Tarro, G.; Perna, A.; Esposito, C. Early diagnosis of lung cancer by detection of tumor liberated protein. J. Cell Physiol 2005, 203, 1–5. [Google Scholar]
- Rey, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Engl. J. Med 2006, 355, 1253–1261. [Google Scholar]
- Al-Hajj, M. From the cover: Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar]
- Kim, C.F.B.; Jackson, E.L.; Woolfenden, A.E.; Lawrence, S.; Babar, I.; Vogel, S.; Crowley, D.; Bronson, R.T.; Jacks, T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005, 121, 823–835. [Google Scholar]
- Ferrari, S.; Harley, V.R.; Pontiggia, A.; Goodfellow, P.N.; Lovell-Badge, R.; Bianchi, M.E. Sry, like hmg1, recognizes sharp angles in DNA. EMBO J 1992, 11, 4497–4506. [Google Scholar]
- Weiss, M.A. Floppy sox: Mutual induced fit in hmg (high-mobility group) box-DNA recognition. Mol. Endocrinol 2001, 15, 353–362. [Google Scholar]
- Pevny, L.H.; Lovell-Badge, R. Sox genes find their feet. Curr. Opin. Genet. Dev 1997, 7, 338–344. [Google Scholar]
- Wegner, M. From head to toes: The multiple facets of sox proteins. Nucleic Acids. Res 1999, 27, 1409–1420. [Google Scholar]
- Wang, Q.; He, W.; Lu, C.; Wang, Z.; Wang, J.; Giercksky, K.E.; Nesland, J.M.; Suo, Z. Oct3/4 and sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinoma. Anticancer Res 2009, 29, 1233–1241. [Google Scholar]
- Scholer, H.R.; Hatzopoulos, A.K.; Balling, R.; Suzuki, N.; Gruss, P. A family of octamer-specific proteins present during mouse embryogenesis: Evidence for germline-specific expression of an oct factor. EMBO J 1989, 8, 2543–2550. [Google Scholar]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Scholer, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the pou transcription factor oct4. Cell 1998, 95, 379–391. [Google Scholar]
- Hay, D.C.; Sutherland, L.; Clark, J.; Burdon, T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells 2004, 22, 225–235. [Google Scholar]
- Boiani, M.; Scholer, H.R. Regulatory networks in embryo-derived pluripotent stem cells. Nat. Rev. Mol. Cell Biol 2005, 6, 872–884. [Google Scholar]
- Monk, M.; Holding, C. Human embryonic genes re-expressed in cancer cells. Oncogene 2001, 20, 8085–8091. [Google Scholar]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar]
- Rodriguez-Pinilla, S.M.; Sarrio, D.; Moreno-Bueno, G.; Rodriguez-Gil, Y.; Martinez, M.A.; Hernandez, L.; Hardisson, D.; Reis-Filho, J.S.; Palacios, J. Sox2: A possible driver of the basal-like phenotype in sporadic breast cancer. Mod. Pathol 2007, 20, 474–481. [Google Scholar]
- Hattab, E.M.; Tu, P.H.; Wilson, J.D.; Cheng, L. Oct4 immunohistochemistry is superior to placental alkaline phosphatase (plap) in the diagnosis of central nervous system germinoma. Am. J. Surg. Pathol 2005, 29, 368–371. [Google Scholar]
- Sanada, Y.; Yoshida, K.; Ohara, M.; Oeda, M.; Konishi, K.; Tsutani, Y. Histopathologic evaluation of stepwise progression of pancreatic carcinoma with immunohistochemical analysis of gastric epithelial transcription factor sox2: Comparison of expression patterns between invasive components and cancerous or nonneoplastic intraductal components. Pancreas 2006, 32, 164–170. [Google Scholar]
- Till, J.E. Stem cells in differentiation and neoplasia. J. Cell Physiol. Suppl 1982, 1, 3–11. [Google Scholar]
- Gangemi, R.M.; Griffero, F.; Marubbi, D.; Perera, M.; Capra, M.C.; Malatesta, P.; Ravetti, G.L.; Zona, G.L.; Daga, A.; Corte, G. Sox2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells 2009, 27, 40–48. [Google Scholar]
- Hu, T.; Liu, S.; Breiter, D.R.; Wang, F.; Tang, Y.; Sun, S. Octamer 4 small interfering rna results in cancer stem cell-like cell apoptosis. Cancer Res 2008, 68, 6533–6540. [Google Scholar]
- Giangreco, A.; Reynolds, S.D.; Stripp, B.R. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am. J. Pathol 2002, 161, 173–182. [Google Scholar]
- Balsara, B.R.; Testa, J.R. Chromosomal imbalances in human lung cancer. Oncogene 2002, 21, 6877–6883. [Google Scholar]
- Hussenet, T.; du Manoir, S. Sox2 in squamous cell carcinoma: Amplifying a pleiotropic oncogene along carcinogenesis. Cell Cycle 2010, 9, 1480–1486. [Google Scholar]
- Sarkaria, I.; O-charoenrat, P.; Talbot, S.G.; Reddy, P.G.; Ngai, I.; Maghami, E.; Patel, K.N.; Lee, B.; Yonekawa, Y.; Dudas, M.; et al. Squamous cell carcinoma related oncogene/dcun1d1 is highly conserved and activated by amplification in squamous cell carcinomas. Cancer Res 2006, 66, 9437–9444. [Google Scholar]
- Idnurm, A.; Hussenet, T.; Dali, S.; Exinger, J.; Monga, B.; Jost, B.; Dembelé, D.; Martinet, N.; Thibault, C.; Huelsken, J.; et al. Sox2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Saito, S.; Onuma, Y.; Ito, Y.; Tateno, H.; Toyoda, M.; Hidenori, A.; Nishino, K.; Chikazawa, E.; Fukawatase, Y.; Miyagawa, Y.; et al. Possible linkages between the inner and outer cellular states of human induced pluripotent stem cells. BMC Syst. Biol 2011, 5 Suppl 1. [Google Scholar] [CrossRef]
- Adachi, K.; Suemori, H.; Yasuda, S.Y.; Nakatsuji, N.; Kawase, E. Role of sox2 in maintaining pluripotency of human embryonic stem cells. Genes Cells 2010, 15, 455–470. [Google Scholar]
- Bernadt, C.T.; Nowling, T.; Rizzino, A. Transcription factor sox-2 inhibits co-activator stimulated transcription. Mol. Reprod. Dev 2004, 69, 260–267. [Google Scholar]
- Huang, P.; Qiu, J.; Li, B.; Hong, J.; Lu, C.; Wang, L.; Wang, J.; Hu, Y.; Jia, W.; Yuan, Y. Role of sox2 and oct4 in predicting survival of hepatocellular carcinoma patients after hepatectomy. Clin. Biochem 2011, 44, 582–589. [Google Scholar]
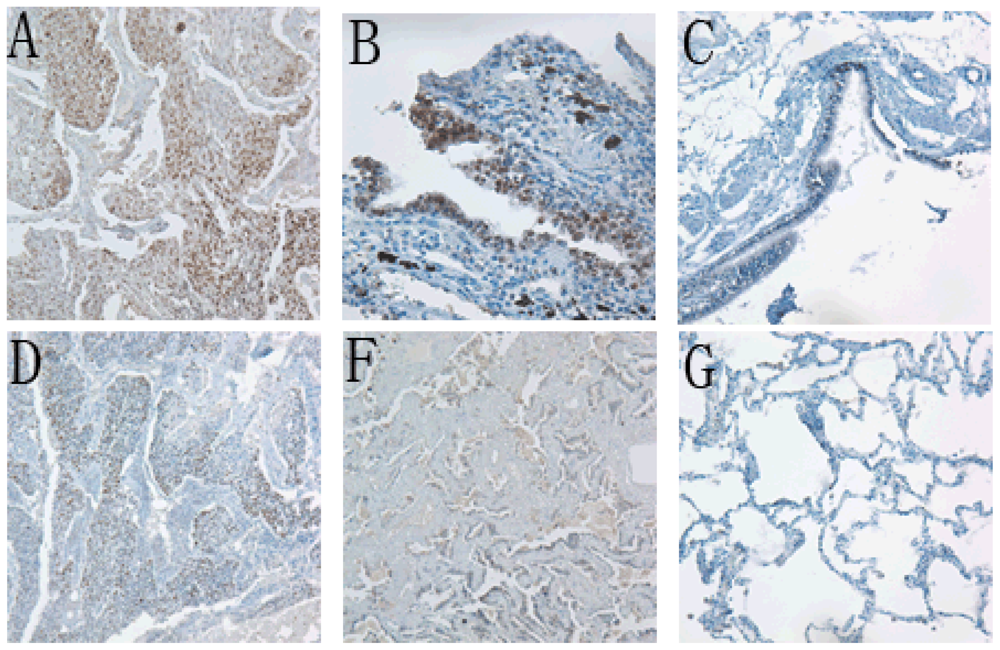


| Cancerous tissues | Paracancerous tissues | Benign tumor tissues | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a Pos | b Neg | Totle | Pos | Neg | Totle | Pos | Neg | Totle | |
| Sox2 | 31 (70.5%) | 13 | 44 | 0 | 44 | 44 | 0 | 21 | 21 |
| Oct4 | 24 (54.5%) | 20 | 44 | 0 | 44 | 44 | 0 | 21 | 21 |
| Variables | Cases | Sox2 | Oct4 | ||||
|---|---|---|---|---|---|---|---|
| a Neg | b Pos | p | Neg | Pos | p | ||
| Age (year) | 0.831 | 0.614 | |||||
| <60 | 18 | 5 | 13 | 9 | 9 | ||
| ≥60 | 26 | 8 | 18 | 11 | 15 | ||
| Gender | 0.340 | 0.757 | |||||
| Male | 33 | 11 | 22 | 15 | 17 | ||
| Female | 11 | 2 | 9 | 5 | 7 | ||
| Location | 0.469 | 0.507 | |||||
| Right | 24 | 6 | 18 | 12 | 12 | ||
| Left | 20 | 7 | 13 | 8 | 12 | ||
| Differentiation | 0.755 | 0.009 * | |||||
| Well | 14 | 5 | 9 | 10 | 4 | ||
| Moderately | 13 | 4 | 9 | 7 | 6 | ||
| Poorly | 17 | 4 | 13 | 3 | 14 | ||
| TNM stage | 0.318 | 0.015 * | |||||
| I~II | 29 | 10 | 19 | 17 | 12 | ||
| III~IV | 15 | 3 | 12 | 3 | 12 | ||
| Pathological type | 0.599 | 0.378 | |||||
| Adnocarcinoma | 23 | 6 | 17 | 9 | 14 | ||
| SCC | 21 | 7 | 14 | 11 | 10 | ||
| Oct4 | Total | ||
|---|---|---|---|
| Sox2 | negative | positive | |
| negative | 5 | 8 | 13 |
| positive | 15 | 16 | 31 |
| Total | 20 | 24 | 44 |
| Gene name | Primer sequence | Size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| Sox2 | F cccccggcggcaatagca R tcggcgccggggagatacat | 448 | 58 |
| Oct4 | F tcccttcgca agccctcat R tgacggtgcagggctccggggaggccc catc | 408 | 55 |
| β-actin | F gatcttgatcttcattgtgctggg R tcgtcaccaactgggacgacatgg | 752 | 55 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, X.; Wang, J.; Xu, Z.; Ahmad, A.; Li, E.; Wang, Y.; Qin, S.; Wang, Q. Expression of Sox2 and Oct4 and Their Clinical Significance in Human Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2012, 13, 7663-7675. https://doi.org/10.3390/ijms13067663
Li X, Wang J, Xu Z, Ahmad A, Li E, Wang Y, Qin S, Wang Q. Expression of Sox2 and Oct4 and Their Clinical Significance in Human Non-Small-Cell Lung Cancer. International Journal of Molecular Sciences. 2012; 13(6):7663-7675. https://doi.org/10.3390/ijms13067663
Chicago/Turabian StyleLi, Xinxin, Jinguang Wang, Zhiyun Xu, Aftab Ahmad, Encheng Li, Yuan Wang, Suli Qin, and Qi Wang. 2012. "Expression of Sox2 and Oct4 and Their Clinical Significance in Human Non-Small-Cell Lung Cancer" International Journal of Molecular Sciences 13, no. 6: 7663-7675. https://doi.org/10.3390/ijms13067663




