Reduction–Oxidation Photocycle Dynamics of Flavins in Starch Films
Abstract
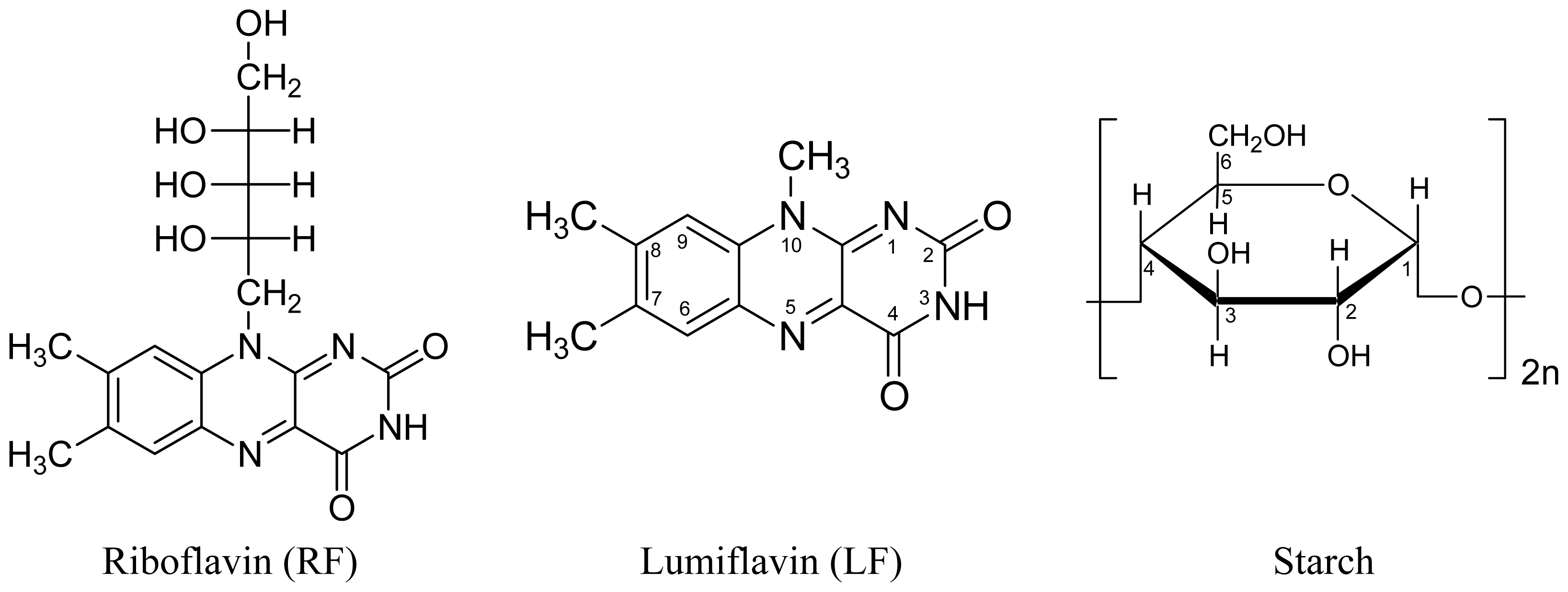
:1. Introduction
2. Results and Discussion
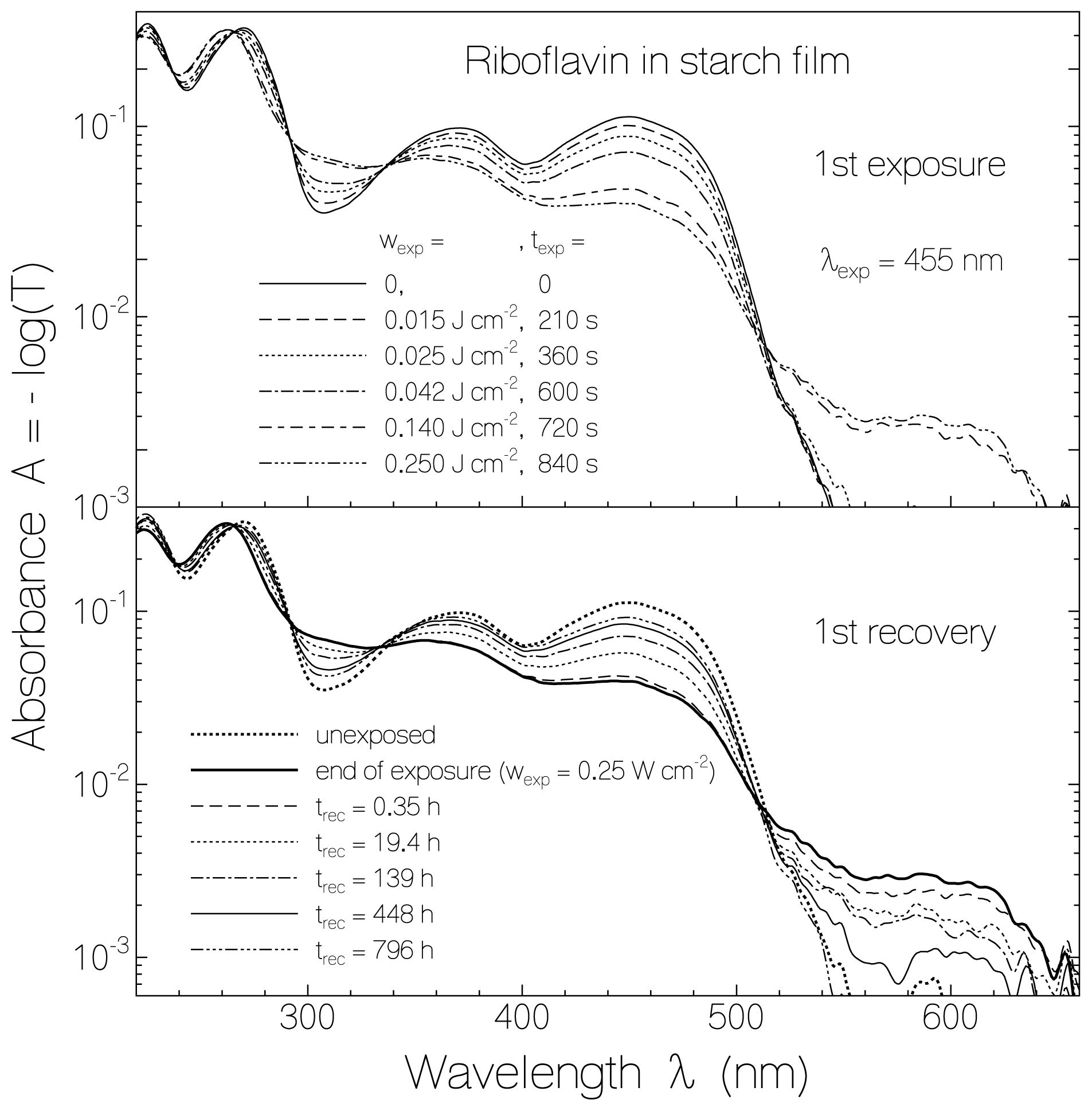
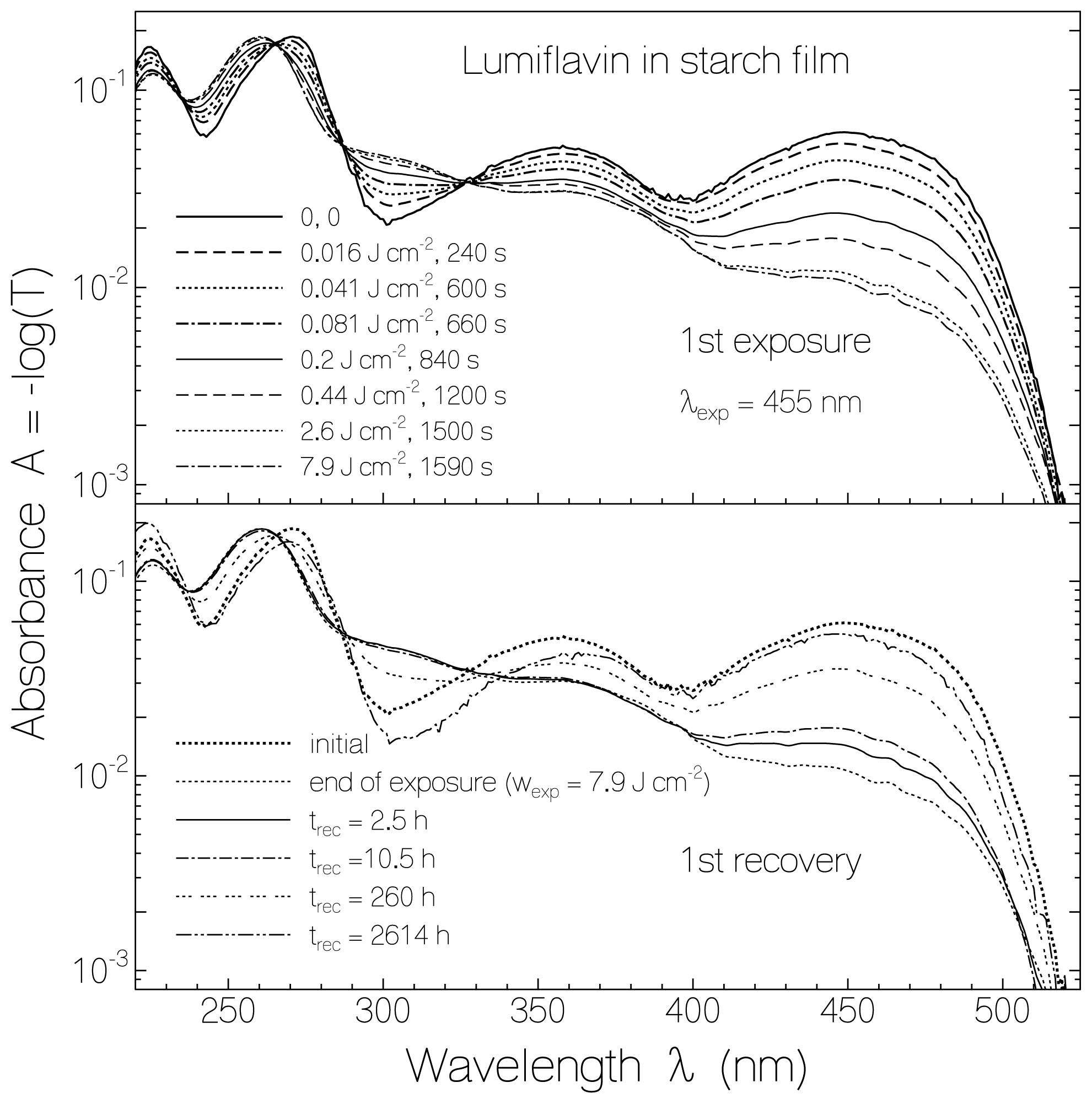
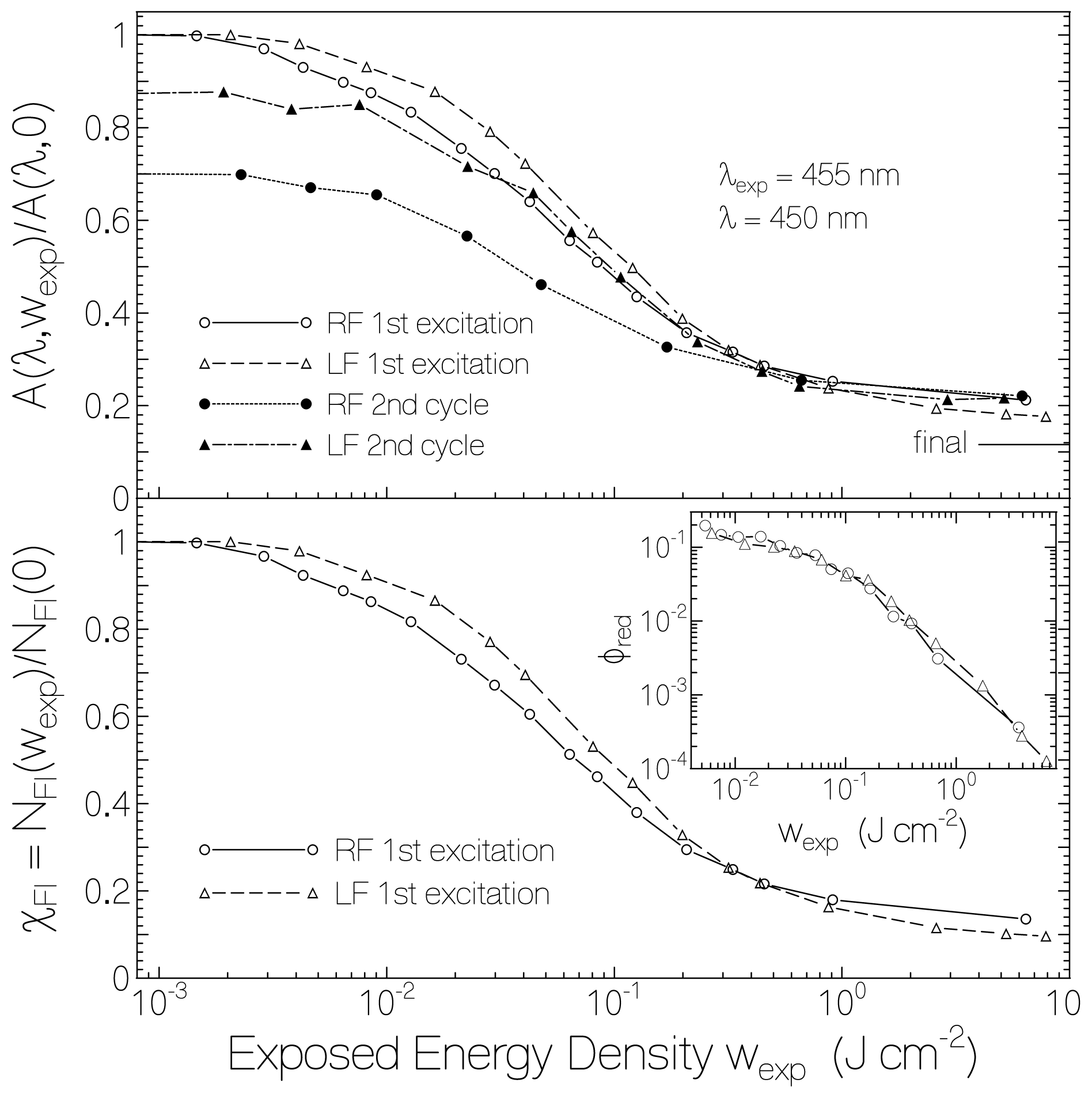
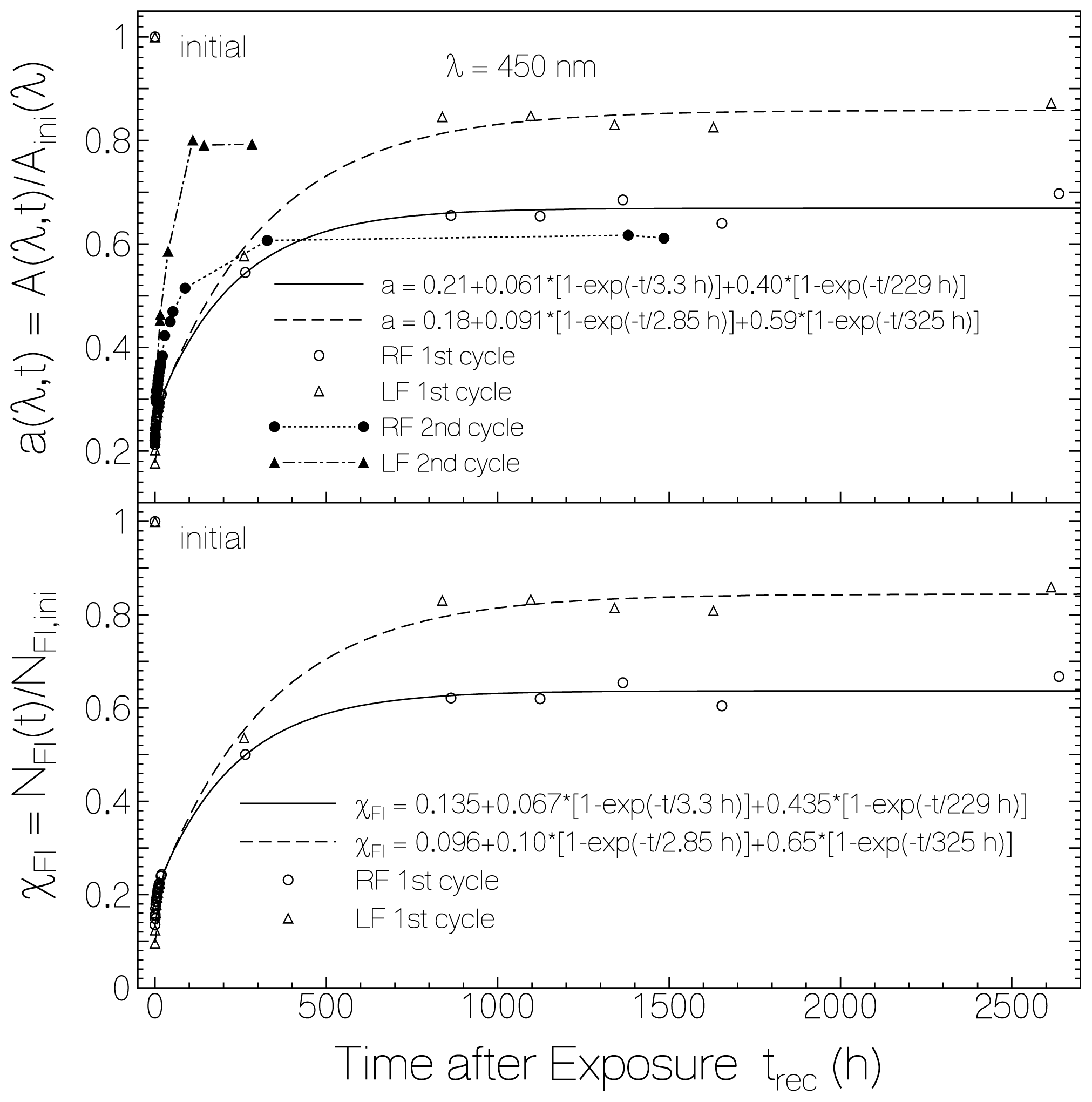
2.1. Absorption Photocycling
2.2. Fluorescence Photocycling
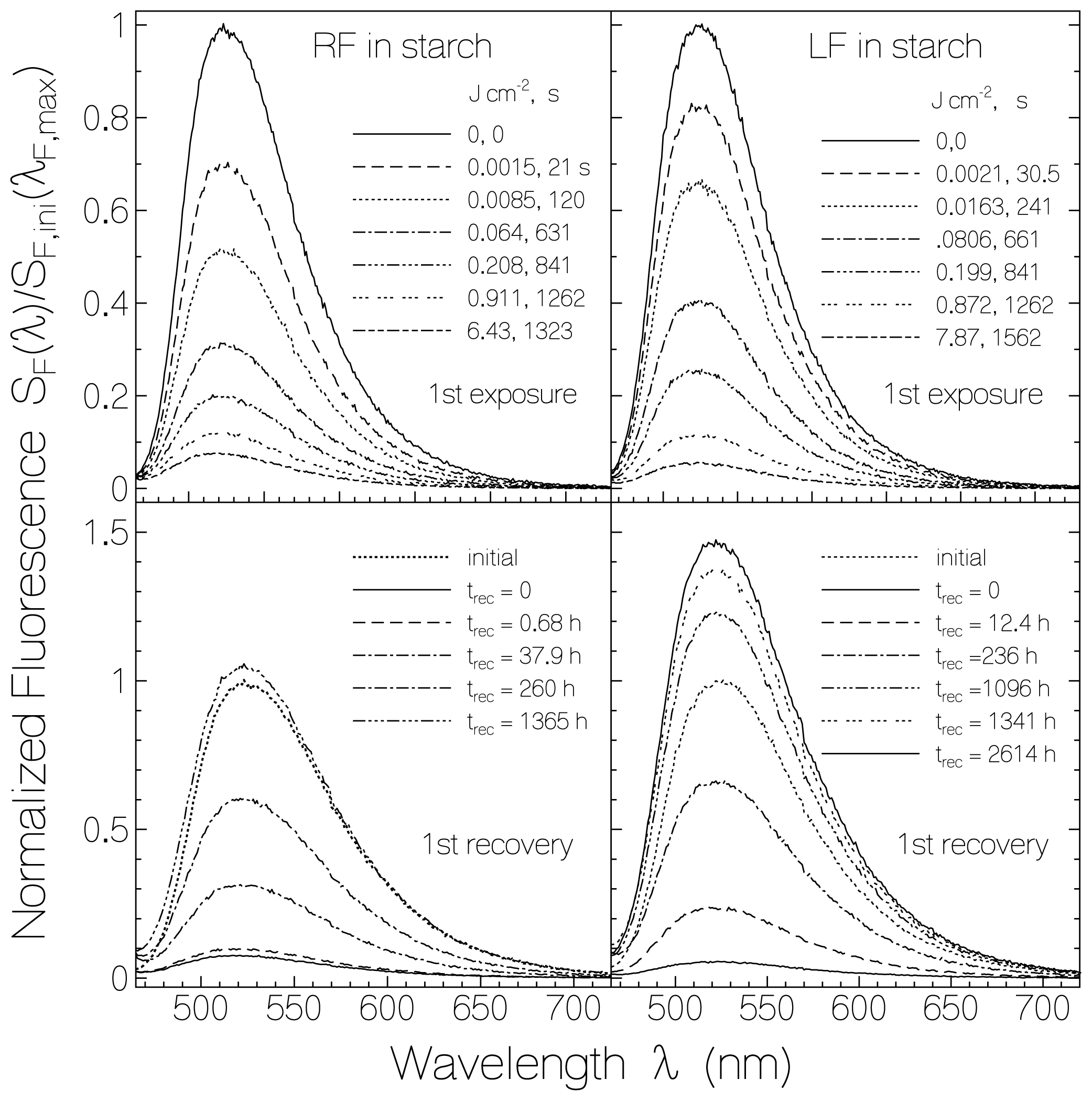
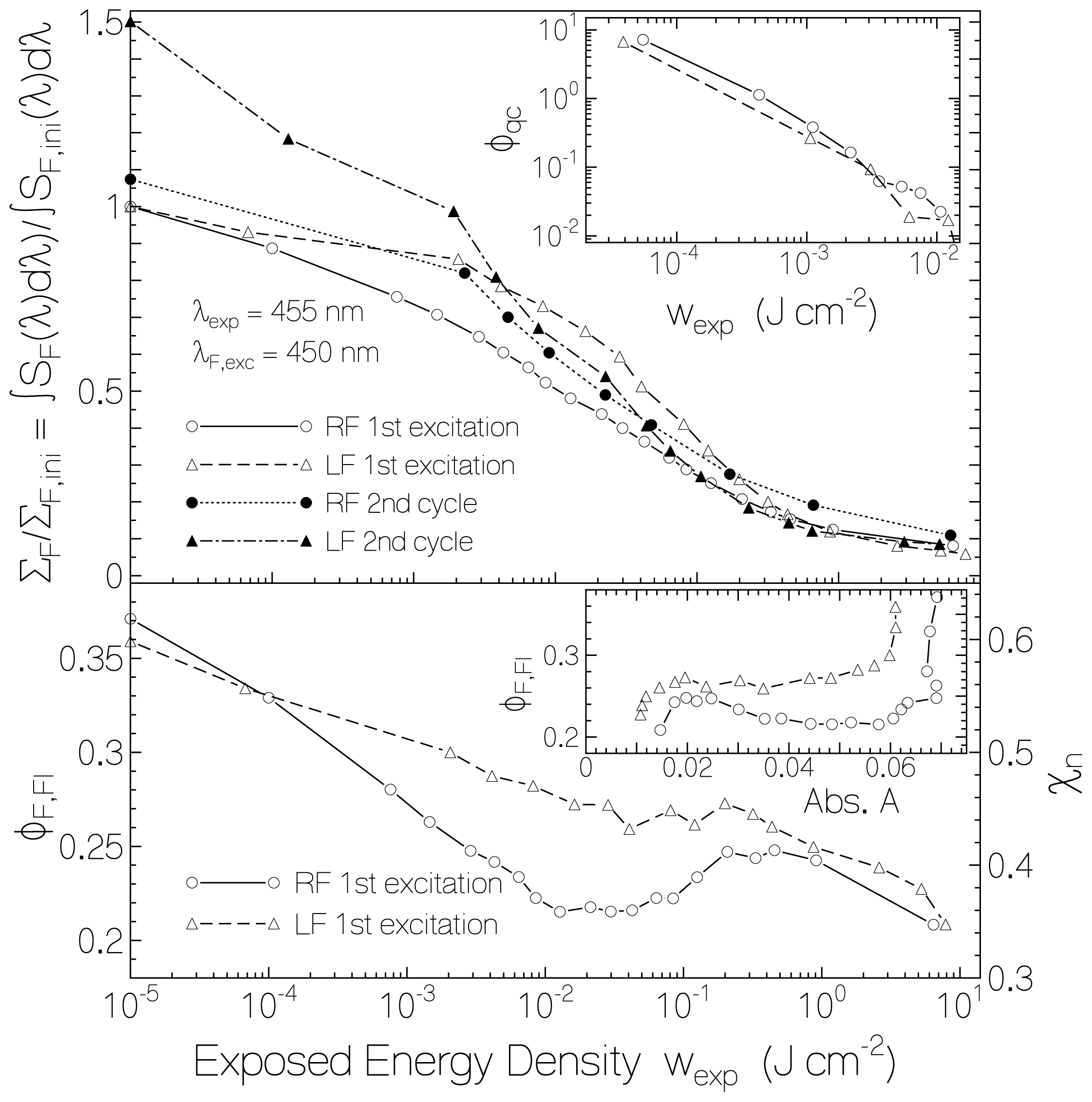
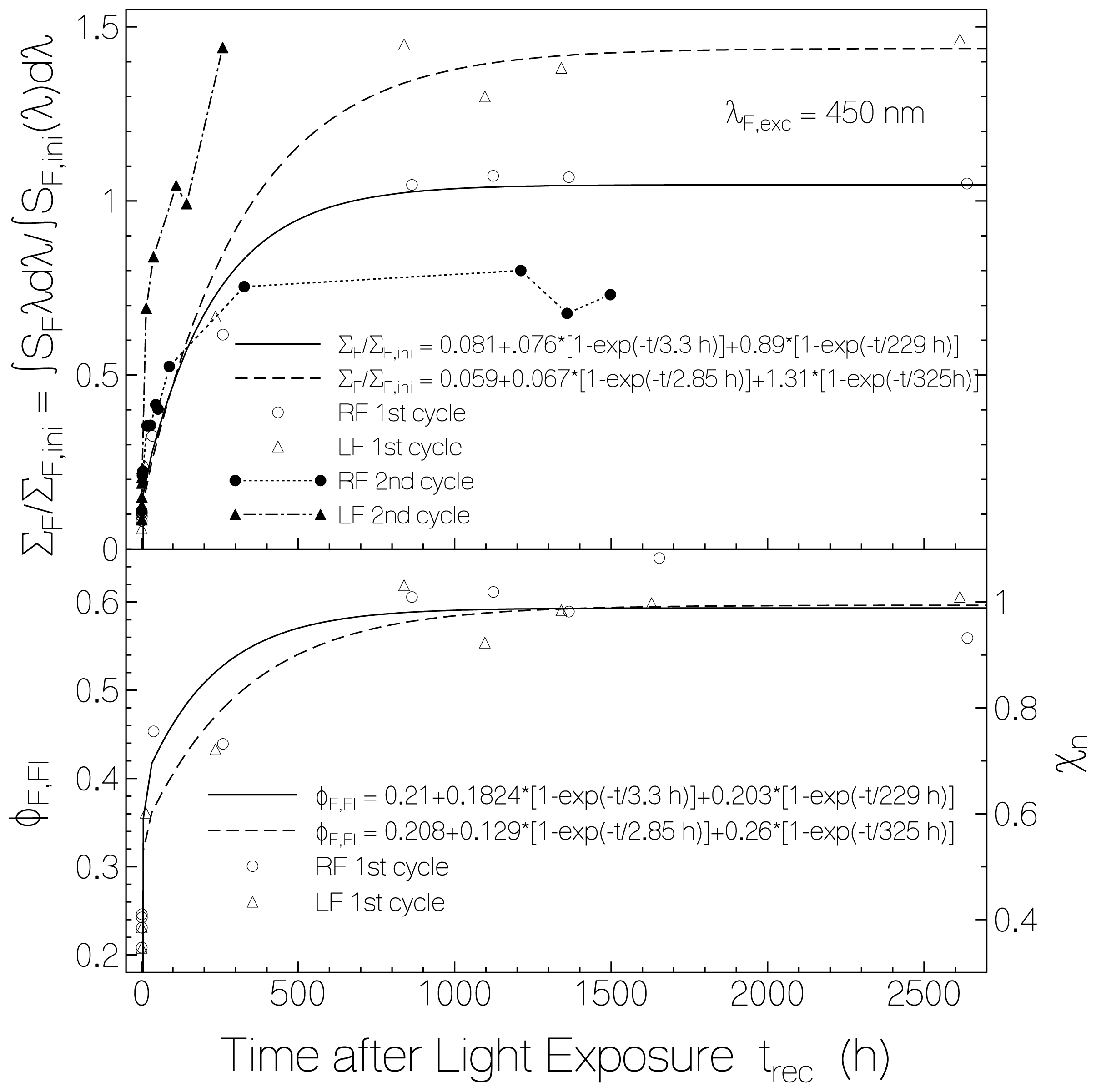
2.2.1. Fluorescence Spectra and Fluorescence Quantum Yields
2.2.2. Fluorescence Lifetimes
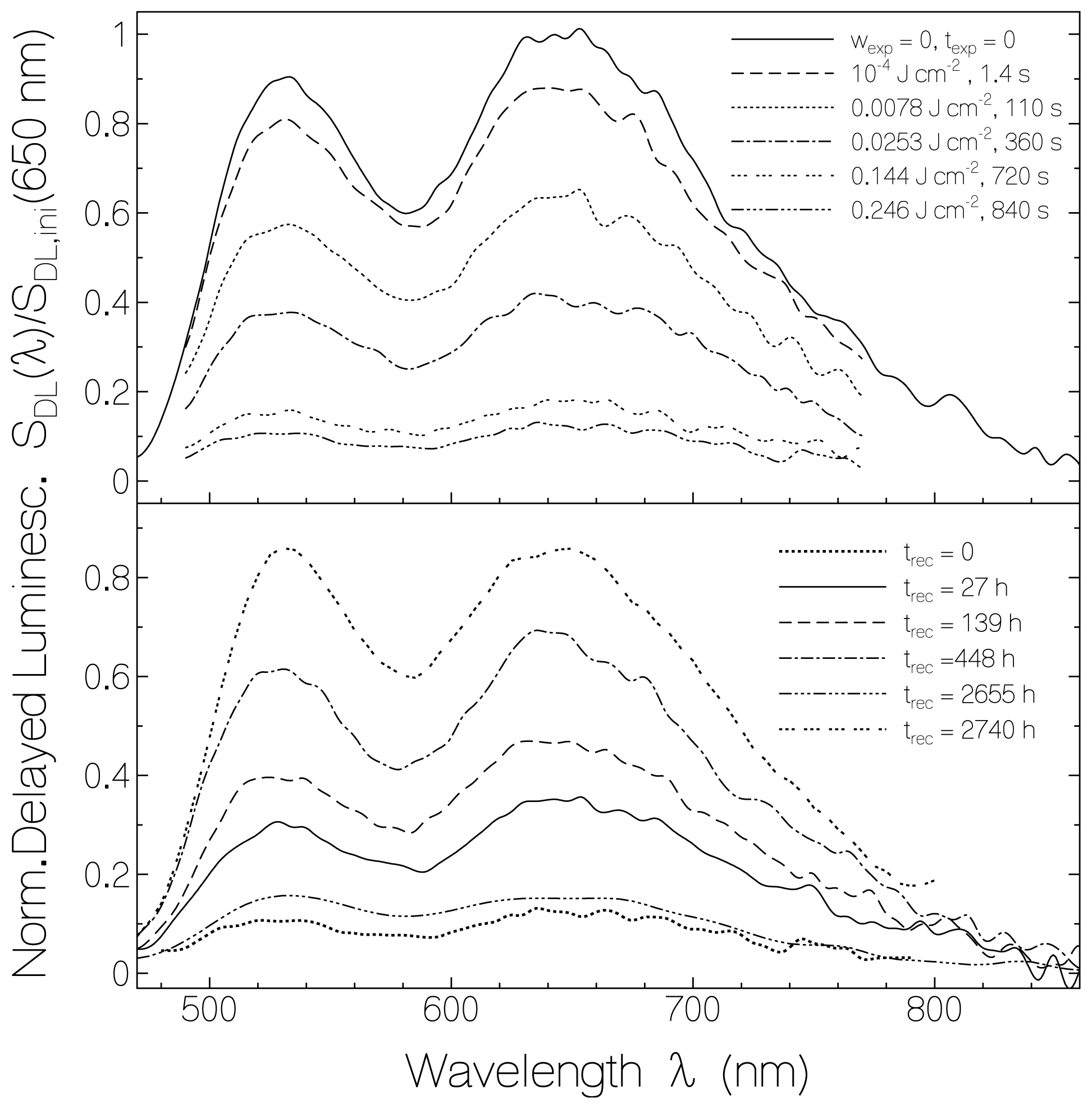
2.3. Delayed Luminescence Studies
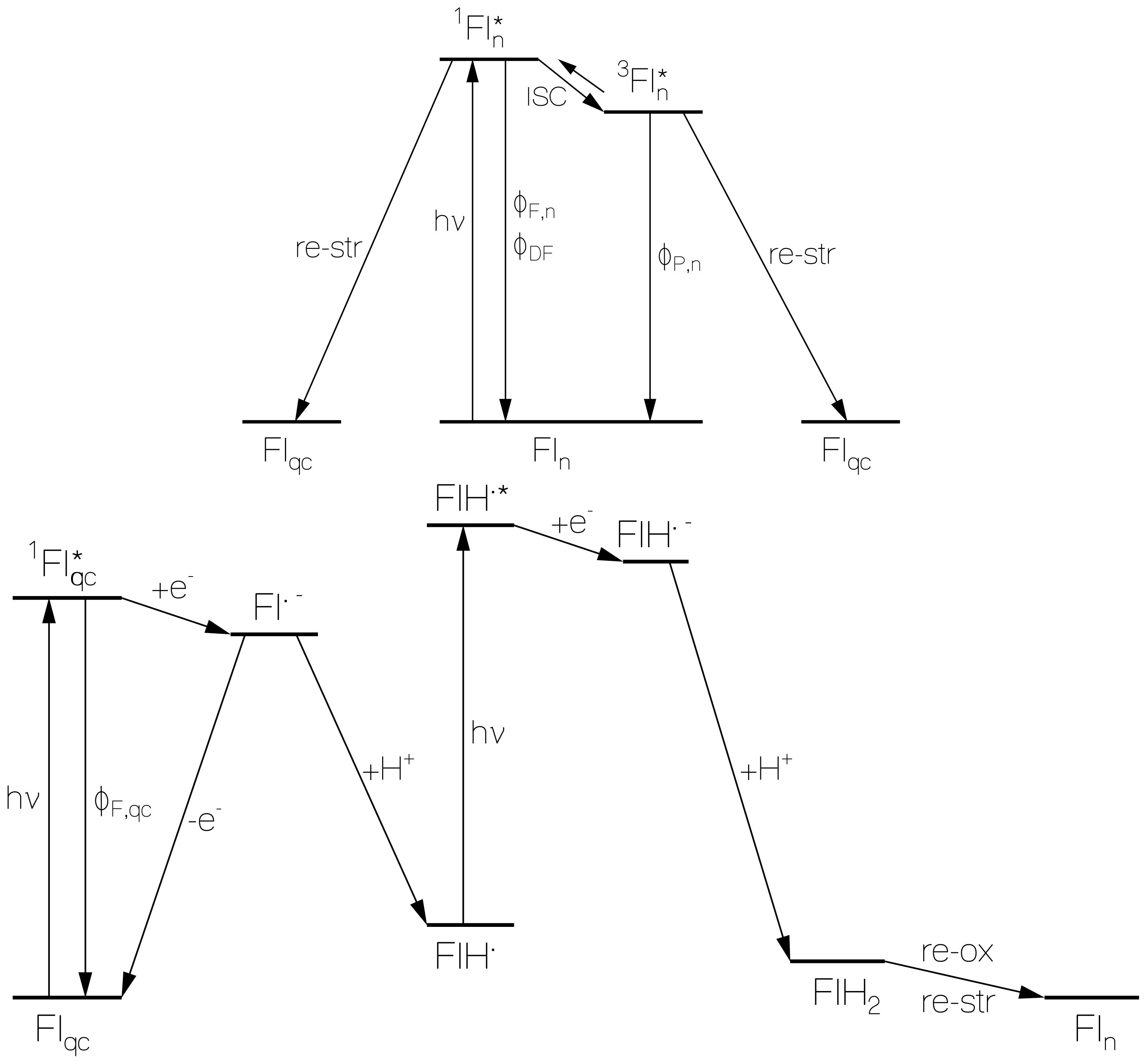
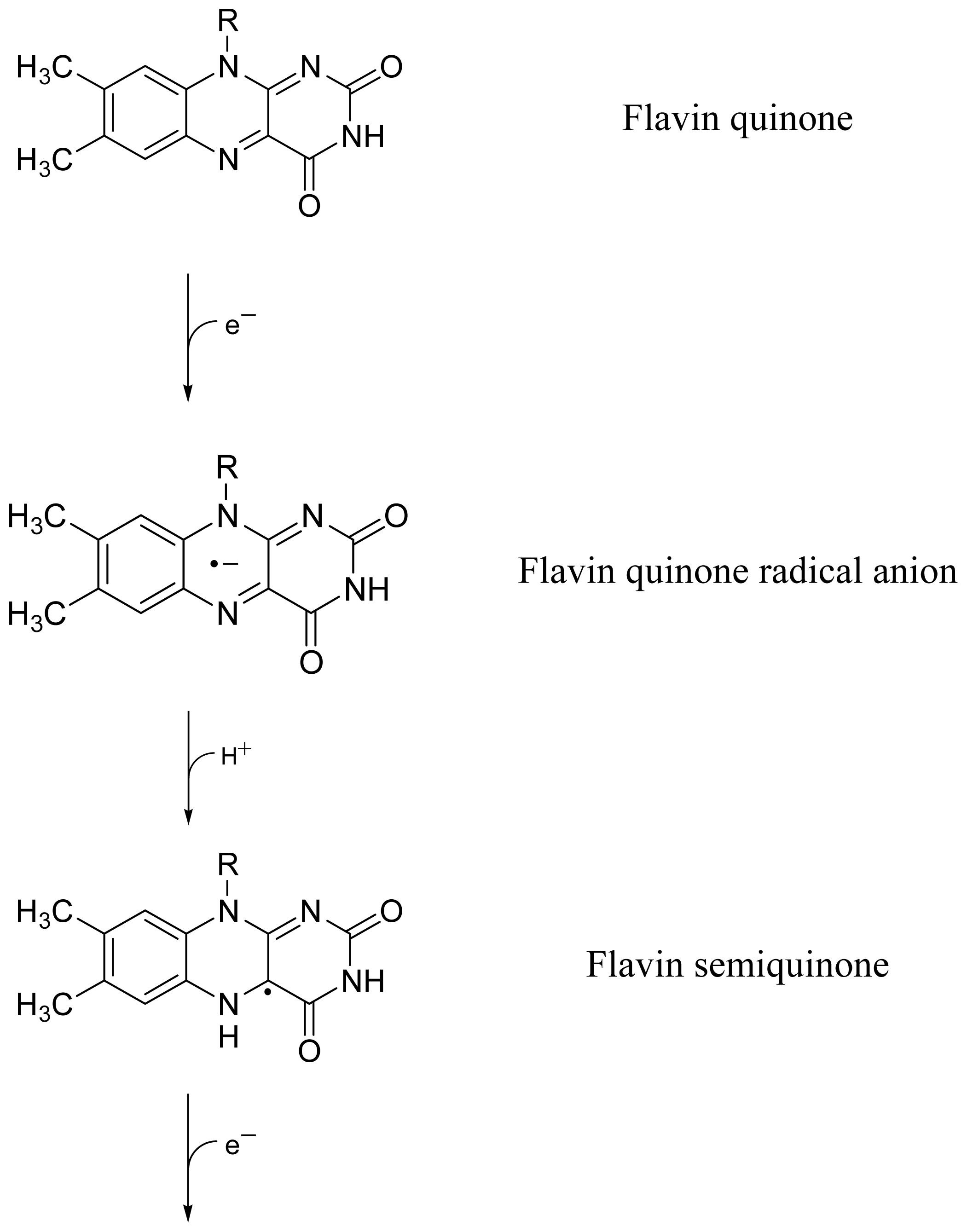
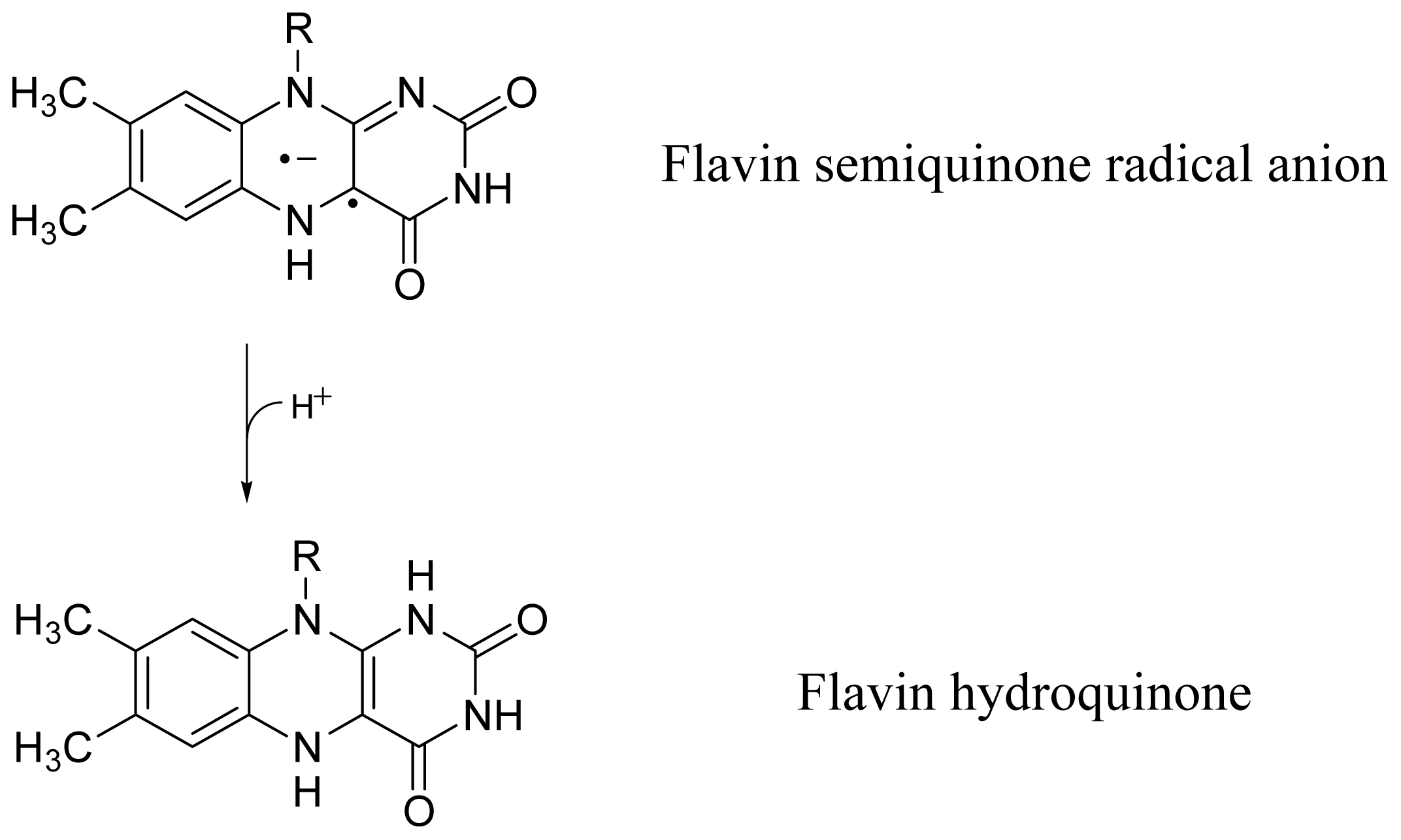
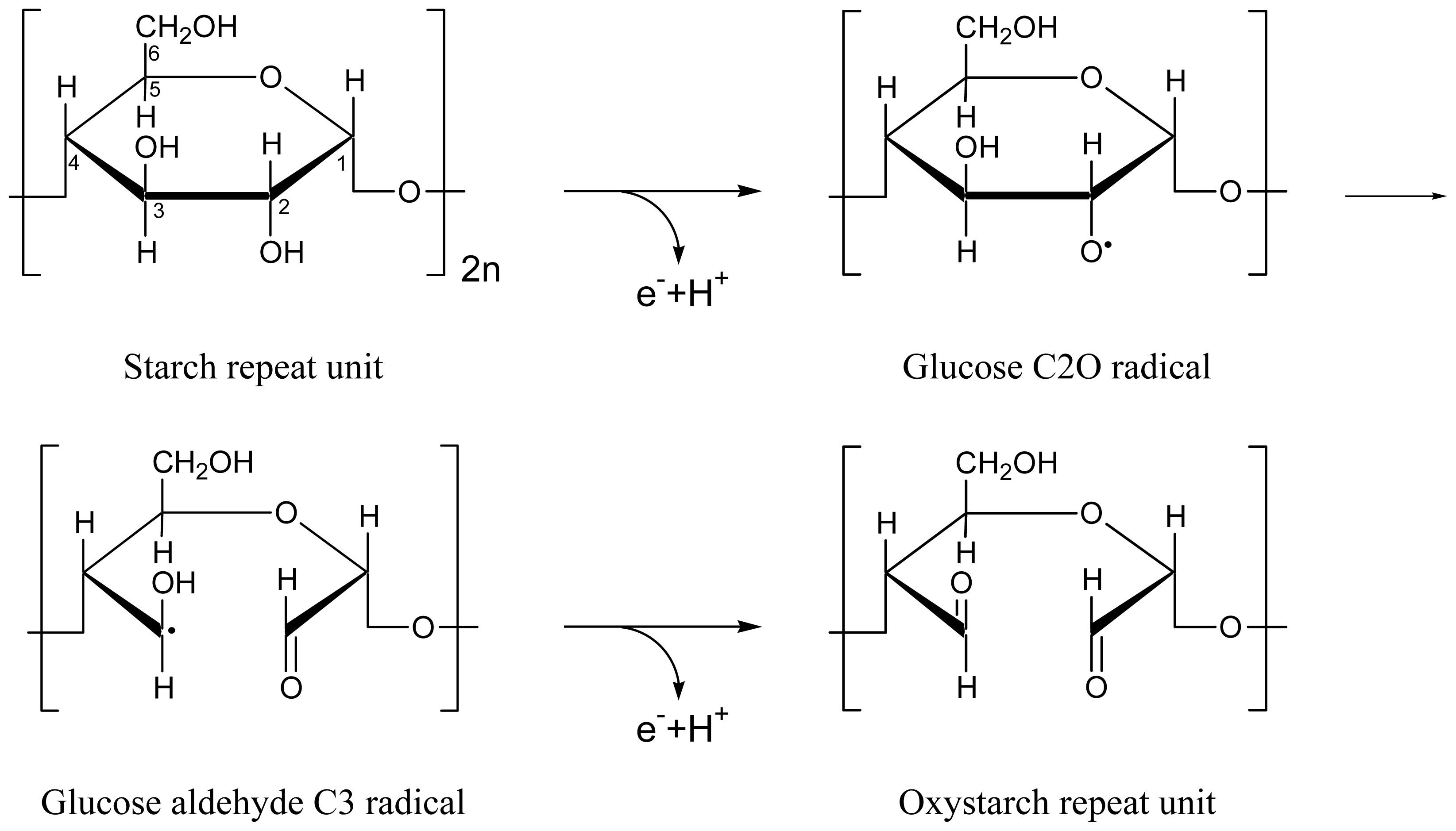
2.4. Discussion of Reduction—Oxidation Photocycle Dynamics
2.5. Comparison with Photocycling of Natural Flavin based Blue-Light Photoreceptors
2.6. Comparison with Dye Reduction and Re-Oxidation by Agents
3. Experimental Section
4. Conclusions
Acknowledgment
References
- Friedrich, W. Vitamins; Walter de Gruyter: Berlin, Germany, 1988. [Google Scholar]
- Müller, F. Chemistry and Biochemistry of Flavoenzymes; CRC Press: Boca Raton, FL, USA, 1991; Volume 1. [Google Scholar]
- Heelis, F. The photophysical and photochemical properties of flavins (isoalloxazines). Chem. Soc. Rev 1982, 11, 15–39. [Google Scholar]
- Silva, E.; Edwards, A.M. Flavins: Photochemistry and Photobiology; Comprehensive Series in Photochemistry and Photobiology; The Royal Society of Chemistry: Cambridge, UK, 2006; Volume 6. [Google Scholar]
- Moore, W.M.; Ireton, R.C. The photochemistry of riboflavin—V. The photodegradation of isoalloxazines in alcoholic solvents. Photochem. Photobiol 1977, 25, 347–356. [Google Scholar]
- Görner, H. Oxygen uptake after electron transfer from amines, amino acids and ascorbic acid to triplet flavins in air-saturated aqueous solution. J. Photochem. Photobiol. B 2007, 87, 73–80. [Google Scholar]
- Zhang, Y.; Görner, H. Flavin-sensitized photo-oxidation of lysozyme and serum albumin. Photochem. Photobiol 2009, 85, 943–948. [Google Scholar]
- Green, M.; Tollin, G. Flash photoysis of flavins. I. Photoreduction in non-aqueous solvents. Photochem. Photobiol 1968, 7, 129–143. [Google Scholar]
- Grodowski, M.S.; Veyret, B.; Weiss, K. Photochemistry of flavins. II. Photophysical properties of alloxazines and isoalloxazines. Photochem. Photobiol 1977, 26, 341–352. [Google Scholar]
- Lasser, N.; Feitelson, J. Excited-state reactions of oxidized flavin derivatives. Photochem. Photobiol 1975, 21, 249–254. [Google Scholar]
- Hemmerich, P.; Knappe, W.R.; Kramer, H.E.A.; Traber, R. Distinction of 2e− and 1e− reduction modes of the flavin chromophore as studied by flash photolysis. Eur. J. Biochem 1980, 104, 511–520. [Google Scholar]
- Batschauer, A. Photoreceptors and Light Signalling; Comprehensive Series in Photochemistry and Photobiology; The Royal Society of Chemistry: Cambridge, UK, 2003; Volume III. [Google Scholar]
- Briggs, W.R.; Spudich, J.L. Handbook of Photosensory Receptors; Wiley-VCH Verlag: Weinheim, Germany, 2005. [Google Scholar]
- Losi, A. Flavin-based blue-light photoreceptors: A photobiophysics update. Photochem. Photobiol 2007, 83, 1283–1300. [Google Scholar]
- Briggs, W.R.; Huala, E. Blue-light photoreceptors in higher plants. Annu. Rev. Cell Dev. Biol 1999, 15, 33–62. [Google Scholar]
- Gomelsky, M.; Klug, G. BLUF: A novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem. Sci 2002, 27, 497–500. [Google Scholar]
- Sancar, A. Structure and function of DNA photolyase and cryptochrome blue-light photorecetors. Chem. Rev 2003, 103, 2203–2237. [Google Scholar]
- Holzer, W.; Penzkofer, A.; Hegemann, P. Photophysical and photochemical excitation and relaxation dynamics of LOV domains of Phot from Chlamydomonas reinhardtii. J. Lumin 2005, 112, 444–448. [Google Scholar]
- Zirak, P.; Penzkofer, A.; Hegemann, P.; Mathes, T. Photo dynamics of BLUF domain mutant H44R of AppA from Rhodobacter sphaeroides. Chem. Phys 2007, 335, 15–27. [Google Scholar]
- Tyagi, A.; Penzkofer, A.; Griese, J.; Schlichting, I.; Kirienko, N.V.; Gomelsky, M. Photodynamics of blue-light-regulated phosphodiesterase BlrP1 protein from Klebsiella pneumonia and its photoreceptor BLUF domain. Chem. Phys 2008, 354, 130–141. [Google Scholar]
- Bouly, J.-B.; Schleicher, E.; Sese, M.D.; Vandenbussche, F.; van der Straeten, D.; Bakrim, N.; Meier, S.; Batschauer, A.; Galland, P.; Bittl, R. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem 2007, 282, 9383–9391. [Google Scholar]
- Banerjee, R.; Schleicher, E.; Meier, S.; Viana, R.M.; Pokorny, R.; Ahmad, M.; Bittl, R.; Batschauer, A. The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J. Biol. Chem 2007, 282, 14916–14922. [Google Scholar]
- Zikihara, K.; Ishikawa, T.; Todo, T.; Tokutomi, S. Involvement of electron transfer in the photoreaction of zebrafish cryptochrome-dash. Photochem. Photobiol 2008, 84, 1016–1623. [Google Scholar]
- Song, S.-H.; Öztürk, N.; Denaro, T.R.; Arat, N.Ö.; Kao, Y.-T.; Zhu, H.; Zhong, D.; Reppert, S.M.; Sancar, A. Formation and function of flavin anion radical in cryptochrome 1 blue-light photoreceptor of monarch butterfly. J. Biol. Chem 2007, 282, 17608–17612. [Google Scholar]
- Zirak, P.; Penzkofer, A.; Moldt, J.; Pokorny, R.; Batschauer, A.; Essen, L.-O. Photocycle dynamics of the E149A mutant of cryptochrome 3 from Arabidopsis thaliana. J. Photochem. Photobiol. B 2009, 97, 94–106. [Google Scholar]
- Immeln, D.; Pokorny, R.; Herman, E.; Moldt, J.; Batschauer, A.; Kottke, T. Photoreaction of plant and DASH cryptochromes probed by infrared spectroscopy: The neutral radical state of flavoproteins. J. Phys. Chem. B 2010, 114, 17155–17161. [Google Scholar]
- Penzkofer, A. Photoluminescence behavior of riboflavin and lumiflavin in liquid solutions and solid films. Chem. Phys 2012, 400, 142–153. [Google Scholar]
- Peat, S.; Bourne, E.J.; Whelan, W.J. Photochemical degradation of starch. Nature 1948, 161, 762–763. [Google Scholar]
- Hofreiter, B.T.; Alexander, B.H.; Wolff, I.A. Rapid estimation of dialdehyde content of periodate oxystarch through quantitative alkali consumption. Anal. Chem 1955, 27, 1930–1931. [Google Scholar]
- Phillips, G.O.; Rickards, T. Photodegradation of carbohydrates. Part IV. Direct photolysis of d-glucose in aqueous solution. J. Chem. Soc. B 1969. [Google Scholar] [CrossRef]
- Bertolini, A.C.; Mestres, C.; Raffi, J.; Buléon, A.; Lerner, D.; Colonna, P. Photodegradation of cassava and corn starches. J. Agric. Food Chem 2001, 49, 675–682. [Google Scholar]
- Drössler, P.; Holzer, W.; Penzkofer, A.; Hegemann, P. Fluorescence quenching of aqueous solutions of riboflavin by methionin and cystein. Chem. Phys 2003, 286, 409–420. [Google Scholar]
- Förster, T. Fluoreszenz Organischer Verbindungen; Vandenhoeck und Ruprecht: Göttingen, Germany, 1951. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed; Springer Verlag: Berlin, Germany, 2006; Volume Chapter 8. [Google Scholar]
- Valeur, B. Molecular Fluorescence. Principles and Applications; Wiley-VCH: Weinheim, Germany, 2002; Volume Chapter 4. [Google Scholar]
- Drössler, P.; Holzer, W.; Penzkofer, A.; Hegemann, P. pH dependence of the absorption and emission behaviour of riboflavin in aqueous solution. Chem. Phys 2002, 282, 429–439. [Google Scholar]
- Islam, S.D.M.; Susdorf, T.; Penzkofer, A.; Hegemann, P. Fluorescence quenching of flavin adenine dinucleotide in aqueous solution by pH dependent isomerisation and photo-induced electron transfer. Chem. Phys 2003, 295, 139–151. [Google Scholar]
- Tyagi, A.; Penzkofer, A. pH dependence of the absorption and emission behaviour of lumiflavin in aqueous solution. J. Photochem. Photobiol. A 2010, 215, 108–115. [Google Scholar]
- Song, S.-H.; Dick, B.; Penzkofer, A.; Hegemann, P. Photo-reduction of flavin mononucleotide to semiquinone form in LOV domain mutants of blue-light receptor Phot from Chlamydomonas reinhardtii. J. Photochem. Photobiol. B 2007, 87, 37–48. [Google Scholar]
- Edmondson, D.E.; Tollin, G. Semiquinone formation in flavo- and metalloflavoproteins. Top. Curr. Chem 1983, 108, 109–138. [Google Scholar]
- Massey, V.; Ghisla, S. Role of charge-transfer interactions in flavoprotein catalysis. Ann. N. Y. Acad. Sci 1974, 227, 446–465. [Google Scholar]
- Ghisla, S. Fluorescence and optical characteristics of reduced flavins and flavoproteins. Methods Enzymol 1980, 66E, 360–373. [Google Scholar]
- Song, S.-H.; Dick, B.; Penzkofer, A. Photo-induced reduction of flavin mononucleotide in aqueous solutions. Chem. Phys 2007, 332, 55–65. [Google Scholar]
- Penzkofer, A.; Stierl, M.; Hegemann, P.; Kateriya, S. Absorption and fluorescence characteristics of photo-activated adenylate cyclase nano-clusters from the amoeboflagellate Naegleria gruberi NEG-M strain. Chem. Phys 2012, 392, 46–54. [Google Scholar]
- Wahl, P.; Auchet, J.C.; Visser, A.J.W.G.; Müller, F. Time resolved fluorescence of flavin adenine dinucleotide. FEBS Lett 1974, 44, 67–70. [Google Scholar]
- Van den Berg, P.A.W.; Feenstra, K.A.; Mark, A.E.; Berendsen, H.J.C.; Visser, A.J.W.G. Dynamic conformations of flavin adenine dinucleotide: Simulated molecular dynamics of the flavin cofactor related to the time-resolved fluorescence characteristics. J. Phys. Chem. B 2002, 106, 8858–8869. [Google Scholar]
- Forssell, P.; Lahtinen, R.; Lahelin, M.; Myllärinen, P. Oxygen permeability of amylose and amylopectin films. Carbonate Polym 2002, 47, 125–129. [Google Scholar]
- Rankin, J.C.; Wolff, I.A.; Davis, H.A.; Rist, C.E. Permeability of amylose film to moisture vapor, selected organic vapors, and the common gases. Ind. Eng. Chem 1958, 3, 120–123. [Google Scholar]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Principles of Molecular Photochemistry. An Introduction; University Science Books: Sausalito, CA, USA, 2009. [Google Scholar]
- Penzkofer, A.; Simmel, M.; Riedl, D. Room temperature phosphorescence lifetime and quantum yield of erythrosine B and rose bengal in aerobic alkaline aqueous solution. J. Lumin 2012, 132, 1055–1062. [Google Scholar]
- Shirdel, J.; Penzkofer, A.; Procházka, R.; Shen, Z.; Daub, J. Absorption and fluorescence spectroscopic characterisation of a phenothiazine-flavin dyad. Chem. Phys 2007, 336, 1–13. [Google Scholar]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev 2003, 103, 1685–1757. [Google Scholar]
- Guillet, J.E.; Andrews, M. Studies of oxygen diffusion in poly(styrene-co-1-naphthyl methacrylate) by phosphorescence quenching. Macromolecules 1992, 25, 2752–2756. [Google Scholar]
- Gao, Y.; Baca, A.M.; Wang, B.; Ogilby, P.R. Activation barriers for oxygen diffusion in polystyrene and polycarbonate glasses: Effects of low molecular weight additives. Macromolecules 1994, 27, 7041–7048. [Google Scholar]
- Holzer, W.; Penzkofer, A.; Susdorf, T.; Álvarez, M.; Islam, S.D.M.; Hegemann, P. Absorption and emission spectroscopic characterisation of the LOV2-domain of Phot from Chlamydomonas reinhardtii fused to a maltose binding protein. Chem. Phys 2004, 302, 105–118. [Google Scholar]
- Masuda, S.; Bauer, C.E. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell 2002, 110, 613–623. [Google Scholar]
- Penzkofer, A.; Stierl, M.; Hegemann, P.; Kateriya, S. Photo-dynamics of the BLUF domain containing soluble adenylate cyclase (nPAC) from the amoeboflagellate Naegleria gruberi NEG-M strain. Chem. Phys 2011, 387, 25–38. [Google Scholar]
- Iseki, M.; Matsunaga, S.; Murakami, A.; Ohno, K.; Shiga, K.; Yoshida, K.; Sugai, M.; Takahashi, T.; Hori, T.; Watanabe, M. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature 2002, 415, 1047–1051. [Google Scholar]
- Zirak, P.; Penzkofer, A.; Lehmpfuhl, C.; Mathes, T.; Hegemann, P. Absorption and emission spectroscopic characterization of blue-light receptor Slr1694 from Synechocystis sp. PCC6803. J. Photochem. Photobiol. B 2007, 86, 22–34. [Google Scholar]
- Shirdel, J.; Zirak, P.; Penzkofer, A.; Breitkreuz, H.; Wolf, E. Absorption and fluorescence spectroscopic characterisation of the circadian blue-light photoreceptor cryptochrome from Drosophila melanogaster (dCry). Chem. Phys 2008, 352, 35–47. [Google Scholar]
- Song, S.-H.; Dick, B.; Penzkofer, A.; Pokorny, R.; Batschauer, A.; Essen, L.-O. Absorption and fluorescence spectroscopic characterization of cryptochrome 3 from Arabidopsis thaliana. J. Photochem. Photobiol. B 2006, 85, 1–16. [Google Scholar]
- Van de Linde, S.; Krstić, I.; Prisner, T.; Doose, S.; Heilmann, M.; Sauer, M. Photoinduced formation of reversible dye radicals and their impact on super-resolution imaging. Photochem. Photobiol. Sci 2011, 10, 499–506. [Google Scholar]
- Heilemann, M.; van de Linde, S.; Mukherjee, A.; Sauer, M. Super-resolution imaging with small organic fluorophores. Angew. Chem. Int. Ed 2008, 48, 6903–6908. [Google Scholar]
- Penzkofer, A.; Bansal, A.K.; Song, S.-H.; Dick, B. Fluorescence quenching of flavins by reductive agents. Chem. Phys 2007, 336, 14–21. [Google Scholar]
- Holmström, B.; Oster, G. Riboflavin as an electron donor in photochemical reactions. J. Am. Chem. Soc 1961, 83, 1867–1871. [Google Scholar]
- Moore, W.M.; Spence, J.T.; Raymond, F.A.; Colson, S.D. Photochemistry of riboflavin. I. The hydrogen transfer process in the anaerobic photobleaching of flavins. J. Am. Chem. Soc 1963, 85, 3367–3372. [Google Scholar]
- Holmström, B. The mechanism of the photoreduction of riboflavin. Arkiv Kemi 1964, 22, 329–346. [Google Scholar]
- Radda, G.K.; Calvin, M. Chemical and photochemical reductions of flavin nucleotides and analogs. Biochemistry 1964, 3, 384–393. [Google Scholar]
- Zulkowsky, K. Verhalten der Stärke gegen Glycerin. Ber. Deutsch. Chem. Ges 1880, 13, 1395–1398. [Google Scholar]
- Zirak, P.; Penzkofer, A.; Mathes, T.; Hegemann, P. Photo-dynamics of roseoflavin and riboflavin in aqueous and organic solvents. Chem. Phys 2009, 358, 111–122. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Penzkofer, A. Reduction–Oxidation Photocycle Dynamics of Flavins in Starch Films. Int. J. Mol. Sci. 2012, 13, 9157-9183. https://doi.org/10.3390/ijms13079157
Penzkofer A. Reduction–Oxidation Photocycle Dynamics of Flavins in Starch Films. International Journal of Molecular Sciences. 2012; 13(7):9157-9183. https://doi.org/10.3390/ijms13079157
Chicago/Turabian StylePenzkofer, Alfons. 2012. "Reduction–Oxidation Photocycle Dynamics of Flavins in Starch Films" International Journal of Molecular Sciences 13, no. 7: 9157-9183. https://doi.org/10.3390/ijms13079157
APA StylePenzkofer, A. (2012). Reduction–Oxidation Photocycle Dynamics of Flavins in Starch Films. International Journal of Molecular Sciences, 13(7), 9157-9183. https://doi.org/10.3390/ijms13079157



