Enhancing Oxidative Stability of Sunflower Oil during Convective and Microwave Heating Using Grape Seed Extract
Abstract
:1. Introduction
2. Results and Discussion
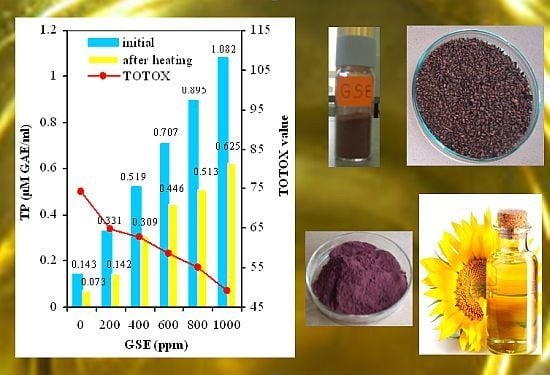
2.1. Evaluation of Antioxidant Properties of GSE
2.2. Impact of Supplementation with GSE and BHT on Oil Quality in the Heating Time
2.2.1. Peroxide Value (PV) and Inhibition of Oil Oxidation (IO)
2.2.2. p-Anisidine Value (p-AV)
2.2.3. Total Oxidation Value (TOTOX Value)
2.2.4. Conjugated Dienes (CD) and Trienes (CT)
2.3. Correlations
3. Experimental Section
3.1. Processing of GSE
3.2. Antioxidant Activity (FRAP Assay)
3.3. Total Phenols Assay
3.4. Application of GSE to Sunflower Oil
3.5. Heating Processes
3.5.1. Convective Heating
3.5.2. Microwave Heating
3.6. Evaluation of Lipid Oxidation
3.7. Statistical Analysis
4. Conclusions
Acknowledgment
- Conflict and InterestThe author declares no conflict of interest.
Abbreviations
| GSE | grape seed extract |
| FRAP | ferric reducing antioxidant power |
| TP | total phenolic |
| PV | peroxide value |
| IO | inhibition of oil oxidation |
| p-AV | p-anisidine value |
| TOTOX value | total oxidation value |
| CD | conjugated dienes |
| CT | conjugated trienes |
| K 232 | specific extinction value at 232 nm |
| K 268 | specific extinction value at 268 nm |
| GAE | gallic acid equivalents |
References
- Gertz, C.; Klosternmann, S.; Kochhar, S.P. Testing and comparing oxidative stability of vegetable oils and fats at frying temperature. Eur. J. Lipid Sci. Tech 2000, 102, 543–551. [Google Scholar]
- El Anany, A.M. Influence of pomegranate (Punica granatum) peel extract on the stability of sunflower oil during deep-fat frying process. Electron. J. Food Plants Chem 2007, 2, 14–19. [Google Scholar]
- Nzikou, J.M.; Matos, L.; Moussounga, J.E.; Ndangui, C.B.; Pambou-Tobi, N.P.; Bandzouzi, E.M.; Kimbonguila, A.; Linder, M.; Desobry, S. Study of oxidative and thermal stability of vegetable oils during frying. Res. J. Appl. Sci 2009, 4, 94–100. [Google Scholar]
- Rehab, F.M.A. Improvement the stability of fried sunflower oil by using different levels of Pomposia (Syzyygium Cumini). Electron. J. Environ. Agric. Food Chem 2010, 9, 396–403. [Google Scholar]
- Albi, T.; Lanzon, A.; Guinda, A.; Perez-Camino, M.C.; Leon, M. Microwave and conventional heating effects on some physical and chemical parameters of edible fats. J. Agric. Food Chem 1997, 45, 3000–3003. [Google Scholar]
- Dostalova, J.; Hanzlik, P.; Reblova, Z.; Pokorny, J. Oxidative changes of vegetable oils during microwave heating. Czech J. Food Sci 2005, 23, 230–239. [Google Scholar]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res 2007, 46, 244–282. [Google Scholar]
- Yanishlieva, N.V.; Marinova, E.M. Stabilization of edible oils with natural antioxidants. Eur. J. Lipid Sci. Technol 2001, 103, 752–767. [Google Scholar]
- Zhang, Y.; Yang, L.; Zu, Y.; Chen, X.; Wang, F.; Liu, F. Oxidative stability of sunflower oil by carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chem 2010, 118, 656–662. [Google Scholar]
- Kalantzakis, G.; Blekas, G. Effect of Greek sage and summer savory extracts on vegetable oil thermal stability. Eur. J. Lipid Sci. Technol 2006, 108, 842–847. [Google Scholar]
- Jeong, S.M.; Kim, S.Y.; Kim, D.R.; Jo, S.C.; Nam, K.C.; Ahn, D.U.; Lee, S.C. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J. Agric. Food Chem 2004, 52, 3389–3393. [Google Scholar]
- Nerantzis, E.; Tataridis, P. Integrated enology-utilisation of winery by-products into high added value products. J. Sci. Technol 2005, 1, 1–12. [Google Scholar]
- Louli, V.; Ragoussis, N.; Magoulas, K. Recovery of phenolic antioxidants from wine industry by-products. Bioresour. Technol 2004, 92, 201–208. [Google Scholar]
- Natella, F.; Belelli, F.; Centili, V.; Ursini, F.; Scaccini, C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J. Agric. Food Chem 2002, 50, 7720–7725. [Google Scholar]
- Shrikhande, A.J. Wine by-products with health benefits. Food Res. Int 2000, 33, 469–474. [Google Scholar]
- Yilmaz, Y.; Toledo, R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Comp. Anal 2006, 19, 41–48. [Google Scholar]
- Jayaprakasha, G.; Selvi, T.; Sakariah, K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int 2003, 36, 117–122. [Google Scholar]
- Lafka, T.I.; Sinanoglou, V.; Lazos, E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem 2007, 104, 1206–1214. [Google Scholar]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem 2001, 73, 285–290. [Google Scholar]
- Bonilla, F.; Mayen, M.; Merida, J.; Medina, M. Extraction of phenolic compounds from red grape marc for use as food lipid antioxidants. Food Chem 1999, 66, 209–215. [Google Scholar]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellapan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem 2003, 51, 5497–5503. [Google Scholar]
- Yemis, O.; Bakkalbasi, E.; Artik, N. Antioxidative activities of grape (Vitis vinifera) seed extracts obtained from different varieties grown in Turkey. Int. J. Food Sci. Technol 2008, 43, 154–159. [Google Scholar]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidative activity from red grape marc extracts. Bioresour. Technol 2003, 87, 431–444. [Google Scholar]
- Mielnik, M.B.; Olsen, E.; Vogt, G.; Adeline, D.; Skrede, G. Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT Food Sci. Technol 2006, 39, 191–198. [Google Scholar]
- Brannan, R.G.; Mah, E. Grape seed extract inhibits lipid oxidation in muscle from different species during refrigerated and frozen storage and oxidation catalyzed by peroxynitrite and iron/ascorbate in a pyrogallol red model system. Meat Sci 2007, 77, 540–546. [Google Scholar]
- Rababah, T.M.; Yucel, S.; Ereifej, K.I.; Alhamad, M.N.; Al-Mahasneh, M.A.; Yang, W.; Muhammad, Al.H.; Ismaeal, K. Effect of grape seed extracts on the physicochemical and sensory properties of corn chips during storage. J. Am. Oil Chem. Soc 2011, 88, 631–637. [Google Scholar]
- Shaker, E.S. Antioxidative effect of extracts from red grape seed and peel on lipid oxidation in oils of sunflower. LWT Food Sci. Technol 2006, 39, 883–892. [Google Scholar]
- Kelen, M.; Tepe, B. Screening of antioxidative properties and total phenolic compounds of various extracts of three different seed of grape varieties (Vitis vinifera L.) from turkish flora. Pakistan J. Biol. Sci 2007, 10, 403–408. [Google Scholar]
- Alexa, E.; Poiana, M.A.; Sumalan, R.M. Mycoflora and ochratoxin A control in wheat grain using natural extracts obtained from wine industry by-products. Int. J. Mol. Sci 2012, 13, 4949–4967. [Google Scholar]
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric 2000, 80, 1925–1941. [Google Scholar]
- Che Man, Y.B.; Liu, J.L.; Jamilah, B.; Rahman, R.A. Quality changes of refined-bleacheddeodorized (RBD) palm olein, soybean oil and their blends during deep-fat frying. J. Food Lipids 1999, 6, 181–193. [Google Scholar]
- Farhoosh, R.; Moosavi, S.M.R. Evaluating the performance of peroxide and conjugated diene values in monitoring quality of used frying oils. J. Agric. Sci. Technol 2009, 11, 173–179. [Google Scholar]
- De Abreu, D.A.P.; Losada, P.P.; Maroto, J.; Cruz, J.M. Evaluation of the effectiveness of a new active packaging film containing natural antioxidants (from barley husks) that retard lipid damage in frozen Atlantic salmon (Salmo salar L.). Food Res. Int 2010, 43, 1277–1282. [Google Scholar]
- Megahed, M.G. Effect of microwave heating of linseed oil on the formation of primary and secondary oxidation products. Agric. Biol. J. N. Am 2011, 2, 673–679. [Google Scholar]
- Erkan, N.; Ayranci, G.; Ayranci, E. A kinetic study of oxidation development in sunflower oil under microwave heating: effect of natural antioxidants. Food Res. Int 2009, 42, 1171–1177. [Google Scholar]
- Suleiman, A.E.M.; El-Makhzangy, A.; Ramadan, M. Antiradical performance and physicochemical characteristics of vegetable oils upon frying of French fries: A preliminary comparative. Electron. J. Environ. Agric. Food Chem 2006, 5, 1429–1441. [Google Scholar]
- Chantzos, N.V.; Georgiou, A.C. Monitoring lipid oxidation events at frying temperatures through radical scavenging assays. Chem. Ind. Chem. Eng. Quart 2007, 13, 163–166. [Google Scholar]
- Benzie, I.F.F.; Strain, L. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem 1996, 239, 70–76. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol 1999, 299, 152–178. [Google Scholar]
- Stef, D.S.; Gergen, I. Effect of mineral-enriched diet and medicinal herbs on Fe, Mn, Zn, and Cu uptake in chicken. Chem. Cent. J 2012, 6, 19. [Google Scholar]
- Mariod, A.A.; Matthäus, B.; Eichner, K.; Hussein, H.I. Antioxidant activity of extracts from Sclerocarya birrea kernel oil cake. Grasas Y Aceites 2006, 57, 361–366. [Google Scholar]
- Tan, C.P.; Che Man, Y.B.; Jinap, S.; Yusoff, M.S.A. Effects of microwave heating on changes in chemical and thermal properties of vegetable oils. J. Am. Oil Chem. Soc 2001, 78, 1227–1232. [Google Scholar]
- Association of Coaching Supervisors, Official and Recommended Practices of the American Oil Chemists’ Society, Official Methods and Recommended Practices, 5th ed; Firestone, D (Ed.) AOAC Press: Champaign, IL, USA, 1998.
- Poiana, M.A.; Alexa, E.; Moigradean, D.; Popa, M. The Influence of the Storage Conditions on the Oxidative Stability and Antioxidant Properties of Sunflower and Pumpkin Oil. Proceedings of the 44th Croatian & 4th International Symposium of Agriculture, Opatija, Croatia, 16–20 February 2009; pp. 449–453.
- Kim, R.; Labella, F. Comparison of analytical methods for monitoring autoxidation profiles of authentic lipids. J. Lipid Res 1987, 28, 1110–1117. [Google Scholar]






| Sample | FRAP value (μmol Fe2+/g) | Total phenolics (μmol GAE/g) |
|---|---|---|
| GSE | 1231.56 ± 17.29 | 1019.83 ± 15.68 |
| BHT | 1339.14 ± 24.93 | - |
| Time (min) | PV (meq/kg oil) | ||||||
|---|---|---|---|---|---|---|---|
| Control | BHT 200 ppm | GSE (ppm) | |||||
| 200 | 400 | 600 | 800 | 1000 | |||
| (a) Convective heating | |||||||
| 0 | 1.77 ± 0.09 a | 1.77 ± 0.09 a | 1.77 ± 0.09 a | 1.77 ± 0.09 a | 1.77 ± 0.09 a | 1.77 ± 0.09 a | 1.77 ± 0.09 a |
| 10 | 4.14 ± 0.12 a | 3.09 ± 0.10 c | 3.78 ± 0.11 b | 3.45 ± 0.18 b | 3.13 ± 0.14 c | 2.72 ± 0.13e | 2.34 ± 0.12 f |
| 20 | 4.60 ± 0.16 a | 3.47 ± 0.18 c | 4.23 ± 0.18 a | 3.89 ± 0.17 b | 3.53 ± 0.12 c | 3.18 ± 0.11 d | 2.46 ± 0.08 e |
| 30 | 5.37 ± 0.16 a | 4.28 ± 0.13 c | 5.08 ± 0.21 a | 4.61 ± 0.23 b | 4.37 ± 0.24 c | 3.47 ± 0.17 d | 2.69 ± 0.18 e |
| 60 | 8.89 ± 0.35 a | 6.30 ± 0.24 c | 8.09 ± 0.19 b | 7.34 ± 0.27 b | 6.13 ± 0.23 c | 5.38 ± 0.26 d | 4.47 ± 0.33 e |
| 120 | 10.01 ± 0.41 a | 7.48 ± 0.26 c | 9.12 ± 0.25 b | 8.25 ± 0.23 b | 7.31 ± 0.33 d | 6.24 ± 0.24 e | 5.19 ± 0.32 f |
| 240 | 12.05 ± 0.76 a | 8.24 ± 0.31 c | 9.81 ± 0.50 b | 9.43 ± 0.59 b | 8.29 ± 0.23 c | 7.31 ± 0.27 d | 6.22 ± 0.32 e |
| (b) Microwave heating | |||||||
| 0 | 1.77 ± 0.09 a | 1.77 ± 0.09 a | 1.77 ± 0.09 a | 1.77 ± 0.09 a | 1.77 ± 0.09 a | 1.77 ± 0.09 a | 1.77 ± 0.09 a |
| 10 | 9.69 ± 0.45 a | 6.27 ± 0.37 d | 9.00 ± 0.43 a | 8.13 ± 0.46 b | 7.12 ± 0.55 c | 6.27 ± 0.35 d | 4.79 ± 0.31 e |
| 20 | 14.73 ± 0.40 a | 10.08 ± 0.47 d | 13.5 ± 0.39 b | 12.3 ± 0.35 c | 10.96 ± 0.37 d | 9.61 ± 0.20 e | 7.09 ± 0.34 f |
| 30 | 19.14 ± 0.61 a | 14.71 ± 0.42 d | 17.59 ± 0.61 b | 16.1 ± 0.34 c | 14.57 ± 0.36 d | 12.36 ± 0.45 e | 9.88 ± 0.39 f |
| 60 | 15.02 ± 0.55 a | 7.59 ± 0.46 d | 12.52 ± 0.41 b | 10.04 ± 0.69 c | 9.38 ± 0.43 c | 7.88 ± 0.53 d | 5.79 ± 0.45 e |
| 120 | 18.81 ± 0.37 a | 14.02 ± 0.38 d | 17.07 ± 0.49 b | 15.7 ± 0.51 c | 15.09 ± 0.50 c | 12.83 ± 0.55 e | 12.24 ± 0.34 f |
| 240 | 16.21 ± 0.38 a | 12.13 ± 0.34 c | 15.56 ± 0.40 a | 14.17 ± 0.58 b | 13.15 ± 0.33 b | 11.97 ± 0.42 c | 11.28 ± 0.17 d |
| Time (min) | p-AV | ||||||
|---|---|---|---|---|---|---|---|
| Control | BHT 200 ppm | GSE (ppm) | |||||
| 200 | 400 | 600 | 800 | 1000 | |||
| (a) Convective heating | |||||||
| 0 | 2.28 ± 0.16 a | 2.28 ± 0.16 a | 2.28 ± 0.16 a | 2.28 ± 0.16 a | 2.28 ± 0.16 a | 2.28 ± 0.16 a | 2.28 ± 0.16 a |
| 10 | 5.27 ± 0.43 a | 3.68 ± 0.31 c | 4.95 ± 0.38 a | 4.56 ± 0.31 a | 4.08 ± 0.36 b | 3.77 ± 0.32 c | 2.81 ± 0.26 d |
| 20 | 9.91 ± 0.54a | 7.47 ± 0.52 c | 9.48 ± 0.61 a | 8.34 ± 0.69 b | 7.85 ± 0.51 c | 7.39 ± 0.56 c | 5.24 ± 0.41 d |
| 30 | 13.3 ± 0.45a | 11.23 ± 0.71 b | 12.79 ± 0.71 a | 12.27 ± 0.79 a | 11.51 ± 0.65 a | 10.24 ± 0.70 c | 8.03 ± 0.50 d |
| 60 | 24.95 ± 1.07 a | 20.81 ± 1.01 b | 23.01 ± 1.04 a | 21.7 ± 1.29 b | 19.79 ± 1.14 c | 17.79 ± 1.08 d | 15.89 ± 1.06 e |
| 120 | 36.24 ± 1.22 a | 31.82 ± 1.36 b | 34.01 ± 1.21 a | 33.28 ± 1.13 a | 31.39 ± 1.33 b | 28.48 ± 1.64 c | 26.04 ± 1.66 d |
| 240 | 50.03 ± 2.01 a | 42.16 ± 1.67 c | 45.25 ± 1.86 b | 43.9 ± 2.14 c | 41.73 ± 1.81 c | 39.18 ± 1.62 d | 35.75 ± 1.44 f |
| (b) Microwave heating | |||||||
| 0 | 2.28 ± 0.16 a | 2.28 ± 0.16 a | 2.28 ± 0.16 a | 2.28 ± 0.16 a | 2.28 ± 0.16 a | 2.28 ± 0.16 a | 2.28 ± 0.16 a |
| 10 | 6.55 ± 0.55 a | 4.82 ± 0.39 b | 5.62 ± 0.41 a | 5.27 ± 0.45 b | 5.03 ± 0.39 b | 4.70 ± 0.41 b | 4.36 ± 0.31 c |
| 20 | 11.64 ± 0.89 a | 8.78 ± 0.70 b | 10.27 ± 0.82 a | 9.46 ± 0.77 b | 9.05 ± 0.74 b | 8.69 ± 0.63 b | 8.02 ± 0.57 c |
| 30 | 19.10 ± 1.68 a | 16.23 ± 1.28 a | 17.66 ± 1.25 a | 16.22 ± 0.88 a | 16.46 ± 1.04 a | 15.96 ± 1.20 a | 14.10 ± 1.06 b |
| 60 | 32.92 ± 2.21 a | 29.67 ± 1.59 a | 31.26 ± 1.28 a | 30.86 ± 1.71 a | 30.35 ± 2.10 a | 29.21 ± 1.82 a | 28.02 ± 1.24 b |
| 120 | 47.93 ± 2.50 a | 43.07 ± 2.03 a | 46.35 ± 2.10 a | 43. 5 ± 2.44 a | 40.13 ± 2.33 b | 38.52 ± 2.09 b | 37.24 ± 1.84 c |
| 240 | 68.80 ± 2.33 a | 50.70 ± 2.89 d | 62.15 ± 3.31 b | 59.69 ± 3.68 c | 50.8 ± 3.46 d | 43.49 ± 2.20 e | 41.36 ± 1.78 f |
| Y = f(X) | Correlation coefficient (r) | |
|---|---|---|
| Convective heating | Microwave heating | |
| TOTOX = f(TP) | −0.985 | −0.986 |
| CD = f(TP) | −0.966 | −0.985 |
| CT = f(TP) | −0.953 | −0.981 |
| PV = f(TP) | −0.972 | −0.983 |
| p-AV = f(TP) | −0.991 | −0.986 |
| IO = f(TP) | 0.976 | 0.994 |
| Heating | Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 10 min | 20 min | 30 min | 60 min | 120 min | 240 min | |
| Convection oven | 25.2 | 151.2 | 171.4 | 181.4 | 185.8 | 190.2 | 192.8 |
| Microwave oven | 26.4 | 144.6 | 157.6 | 178.2 | 181.4 | 185.6 | 190.2 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Poiana, M.-A. Enhancing Oxidative Stability of Sunflower Oil during Convective and Microwave Heating Using Grape Seed Extract. Int. J. Mol. Sci. 2012, 13, 9240-9259. https://doi.org/10.3390/ijms13079240
Poiana M-A. Enhancing Oxidative Stability of Sunflower Oil during Convective and Microwave Heating Using Grape Seed Extract. International Journal of Molecular Sciences. 2012; 13(7):9240-9259. https://doi.org/10.3390/ijms13079240
Chicago/Turabian StylePoiana, Mariana-Atena. 2012. "Enhancing Oxidative Stability of Sunflower Oil during Convective and Microwave Heating Using Grape Seed Extract" International Journal of Molecular Sciences 13, no. 7: 9240-9259. https://doi.org/10.3390/ijms13079240