Correlation among Antioxidant, Antimicrobial, Hemolytic, and Antiproliferative Properties of Leiothrix spiralis Leaves Extract
Abstract
:1. Introduction
2. Results and Discussion
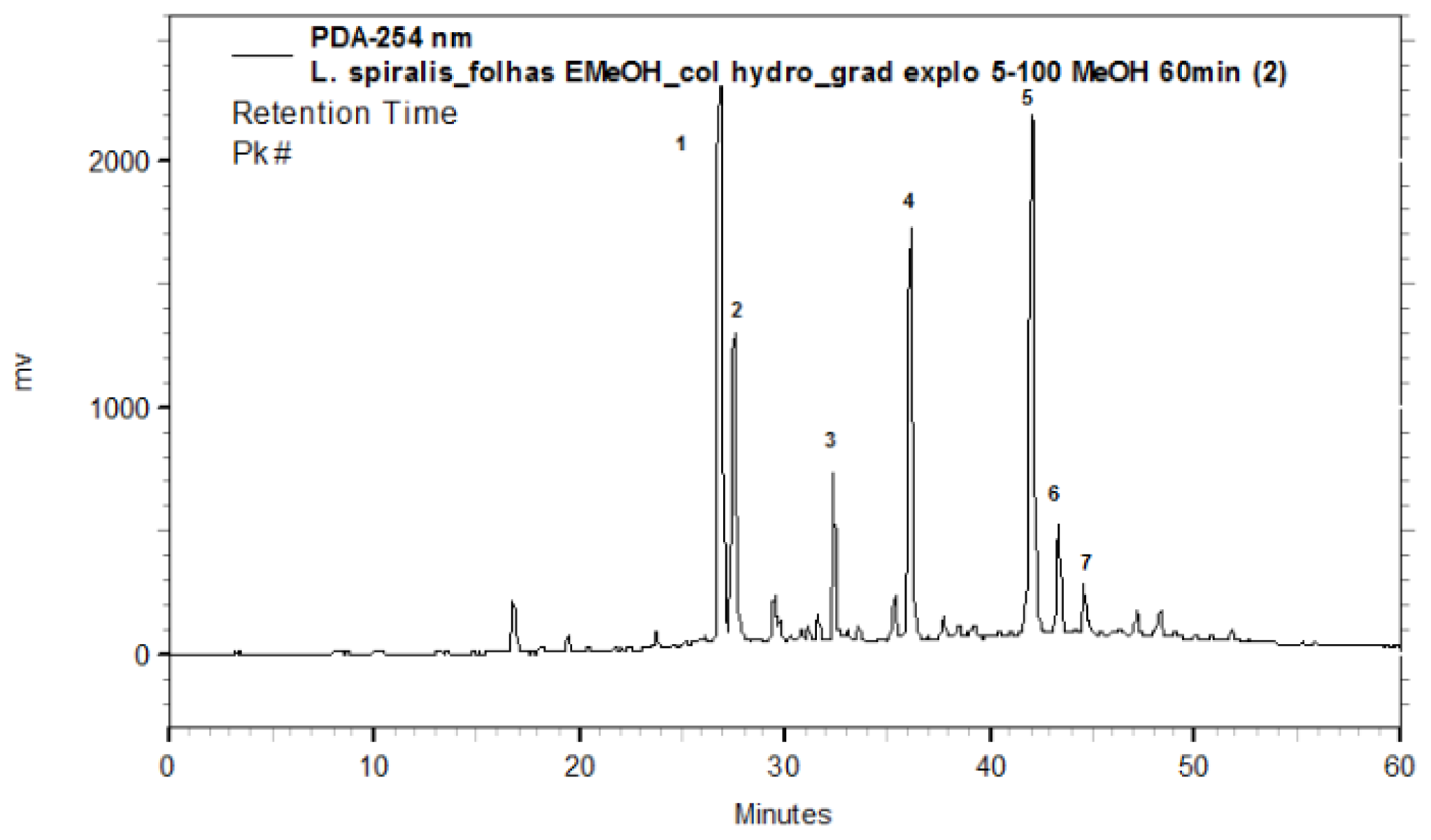
2.1. Chemical Characterization
2.2. Total Flavonoids
2.3. ABTS Radical Cation Scavenging Activity
2.4. Antibacterial and Antifungal Susceptibility
2.5. Inhibition of Hyphal Formation
2.6. MTT and LDH Cell Viability
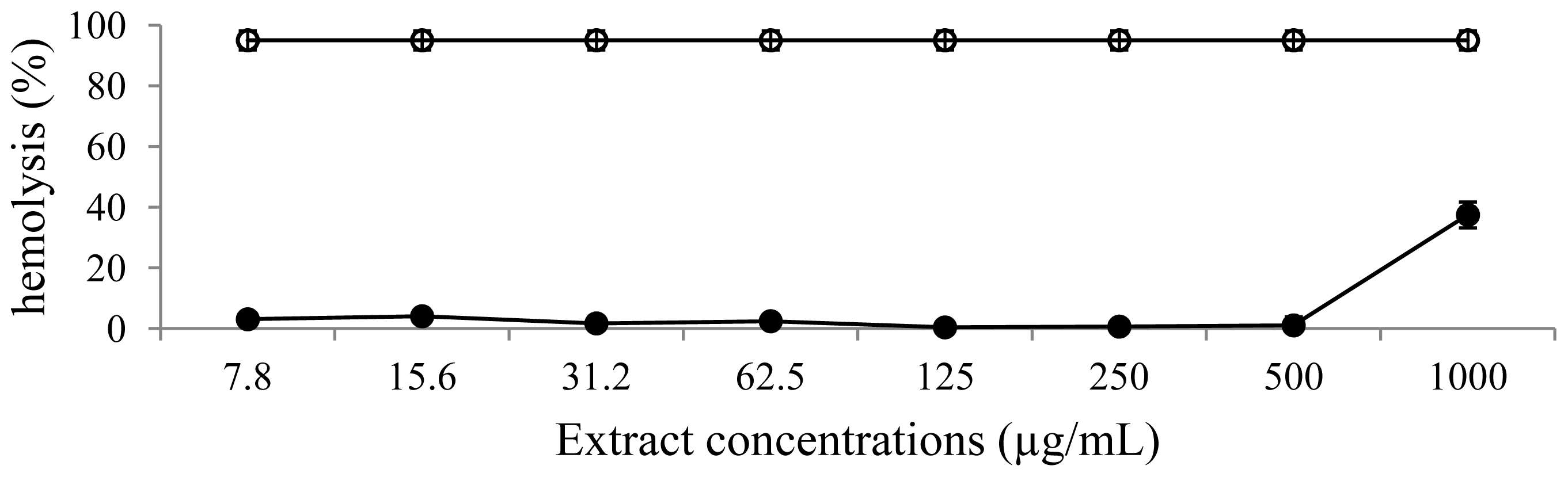
2.7. Hemolytic Assay
3. Experimental Section
3.1. Plant Material
3.2. Chemical Characterization of the Extract
3.3. Total Flavonoids
3.4. ABTS Radical Cation Scavenging Activity
3.5. Antimicrobial Activity
3.6. Inhibition of Hyphal Growth
3.7. MTT and LDH Cell Viability Assay
3.8. Hemolytic Assay
4. Conclusions
Acknowledgments
References
- Waris, G.; Ahsan, H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J. Carcinog 2006, 5, 1–8. [Google Scholar]
- Hu, H.; Zhang, Z.; Lei, Z.; Yang, Y.; Sugiura, N. Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. J. Biosci. Bioeng 2009, 107, 42–48. [Google Scholar]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza vírus. Antivir. Res 2005, 68, 66–74. [Google Scholar]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar]
- Silva, M.A.; Cardoso, C.A.L.; Vilegas, W.; Santos, L.C. High-performance liquid chromatographic quantification of flavonoids in Eriocaulaceae species and their antimicrobial activity. Molecules 2009, 14, 4644–4654. [Google Scholar]
- Ferguson, P.J.; Kurowska, E.; Freeman, D.J.; Chambers, A.F.; Koropatnick, D.J. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J. Nutr 2004, 134, 1529–1535. [Google Scholar]
- Russo, A.; Cardile, V.; Sanchez, F.; Troncoso, N.; Vanella, A.; Garbarino, J.A. Chilean propolis: antioxidant activity and antiproliferative action in human tumor cell lines. Life Sci 2004, 76, 545–558. [Google Scholar]
- Boivin, D.; Lamy, S.; Lord-Dufour, S.; Jackson, J.; Beaulieu, E.; Côté, M.; Moghrabi, A.; Barrette, S.; Gingras, D.; Béliveau, R. Antiproliferative and antioxidant activities of common vegetables: A comparative study. Food Chem 2009, 112, 374–380. [Google Scholar]
- Hu, W.; Yu, L.; Wanga, M.H. Antioxidant and antiproliferative properties of water extract from Mahonia bealei (Fort.) Carr. leaves. Food Chem. Toxicol 2010, 49, 799–806. [Google Scholar]
- Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Gonzalez-Laredo, R.F.; Reynoso-Camacho, R.; Ramos-Gomez, M.; García-Gasca, T.; Rodríguez-Muñoz, E.; Guzmán-Maldonado, S.H.; Medina-Torres, L.; Luján-García, B.A. Antioxidant activity and genotoxic effect on HeLa cells of phytophenolic compounds from infusions of Quercus resinosa leaves. Food Chem 2009, 115, 1320–1325. [Google Scholar]
- Sano, P.T. Actinocephalus (Koern.) Sano (Paepalanthus sect. Actinocephalus), a new genus of Eriocaulaceae, and other taxonomic and nomenclatural changes involving Paepalanthus. Mart Taxon 2004, 53, 99–107. [Google Scholar]
- Salatino, A.; Salatino, M.L.; Giulietti, A.M. Contents of soluble phenolic compounds of capitula of Eriocaulaceae. Quim. Nova 1990, 13, 289–292. [Google Scholar]
- Dokkedal, A.L.; Salatino, A. Flavonoids of brazilian species of Leiothrix (Eriocaulacaeae). Biochem. System. Ecol 1992, 20, 31–32. [Google Scholar]
- Santos, L.C.; Piacente, S.; Montoro, P.; Pizza, C.; Vilegas, W. Atividade antioxidante de xantonas isoladas de espécies de Leiothix (Eriocaulaceae). Rev. Bras. Farmacogn 2003, 13, 67–74. [Google Scholar]
- Araújo, M.G.F.; Hilário, F.; Nogueira, L.G.; Vilegas, W.; Santos, L.C.; Bauab, T.M. Chemical constituents of the methanolic extract of leaves of Leiothrix spiralis ruhland and their antimicrobial activity. Molecules 2011, 16, 10479–10490. [Google Scholar]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The ultraviolet spectra of flavones and flavonols. In The Systematic Identification of Flavonoids; Mabry, T.J., Markham, K.R., Thomas, M.B., Eds.; Springer-Verlag: New York, USA, 1970; Volume 41–45, pp. 165–166. [Google Scholar]
- Cheng, L.X.; Tang, J.J.; Luo, H.; Jin, X.L.; Dai, F.; Yang, J.; Qian, Y.P.; Li, X.Z.; Zhou, B. Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorg. Med. Chem. Lett 2010, 20, 2417–2420. [Google Scholar]
- Parry, J.; Su, L.; Moore, J.; Cheng, Z.; Luther, M.; Rao, J.N.; Wang, J.Y.; Yu, L. Chemical compositions, antioxidant capacities, and antiproliferative activities of selected seed flours. J. Agr. Food Chem. 2006, 54, 3773–3778. [Google Scholar]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agr. Food Chem 2008, 56, 670–675. [Google Scholar]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. Food Sci. Technol 2009, 42, 1–8. [Google Scholar]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H-M. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr. Cancer Drug Targets. 2008, 8, 634–646. [Google Scholar]
- Cowan, N.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev 1999, 12, 564–582. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar]
- Tamaki, M.; Imazeki, Y.; Shirane, A.; Fujinuma, K.; Shindo, M.; Kimura, M.; Uchida, Y. Novel gratisin derivatives with high antimicrobial activity and low hemolytic activity. Bioorg. Med. Chem. Lett 2011, 21, 440–443. [Google Scholar]
- Singh, R.P.; Kaur, G. Hemolytic activity of aqueous extract of Livistona chinensis fruits. Food Chem. Toxicol 2008, 46, 553–556. [Google Scholar]
- Kitagawa, S.; Sakamoto, H.; Tano, H. Inhibitory Effects of Flavonoids on Free Radical-Induced Hemolysis and Their Oxidative Effects on Hemoglobin. Chem Pharm Bull 2004, 52, 999–1001. [Google Scholar]
- Asgary, S.; Naderi, G.H.; Askari, N. Protective effect of flavonoids against red blood cell hemolysis by free radicals. Exp. Clin. Cardiol 2005, 10, 88–90. [Google Scholar]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Piatti, E. Honey flavonoids as protection agents against oxidative damage to human red blood cells. Food Chem 2007, 104, 1635–1640. [Google Scholar]
- Yang, Y.L. Virulence factors of Candida species. J. Microbiol. Immunol. Infect 2003, 36, 223–228. [Google Scholar]
- Knobloch, E.; Pauli, A.; Iberl, B.; Wies, N.; Weigand, H. Mode of action of essential oil components on whole cells of bacteria-and fungi in plate tests. In Bioflavour: Analyses, Biochemistry, Biotechnology; Schreier, P., Ed.; Walter de Gruyter: Berlin, Germany, 1988; pp. 287–299. [Google Scholar]
- Dambolena, J.S.; Zunino, M.P.; López, A.G.; Rubinstein, H.R.; Zygadlo, J.A.; Mwangi, J.W.; Thoithi, G.N.; Kibwage, I.O.; Mwalukumbi, J.M.; Kariuki, S.T. Essential oils composition of Ocimum basilicum L. and Ocimum gratissimum L. from Kenya and their inhibitory effects on growth and fumonisin production by Fusarium verticillioides. Inn. Food. Sci. Emerging. Technol 2010, 11, 410–414. [Google Scholar]
- Dambolena, J.S.; Zygadlo, J.A.; Rubinstein, H.R. Antifumonisin activity of natural phenolic compounds. A structure-property-activity relationship study. Int. J. Food Microbiol 2011, 145, 140–146. [Google Scholar]
- Lassen, N.; Black, W.J.; Estey, T.; Vasiliou, V. The role of corneal crystallins in the cellular defense mechanisms against oxidative stress. Semin. Cell. Dev. Biol 2008, 19, 100–112. [Google Scholar]
- Cutter, H.; Wu, L-Y.; Kim, C.; Morre, D.J.; Morre, D.M. Is the cancer protective effect correlated with growth inhibitions by green tea (–)-epigallocatechin gallate mediated through an antioxidant mechanism? Cancer Lett 2001, 162, 149–154. [Google Scholar]
- García-Alonso, J.; Ros, G.; Periago, M.J. Antiproliferative and cytoprotective activities of a phenolic-rich juice in HepG2 cells. Food Res. Int 2006, 39, 982–991. [Google Scholar]
- Luo, W.; Zhao, M.; Yang, B.; Ren, J.; Shen, G.; Rao, G. Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chem 2011, 126, 277–282. [Google Scholar]
- Lazzè, M.C.; Savio, M.; Pizzala, R.; Cazzalini, O.; Perucca, P.; Scovassi, A.I.; Stivala, L.A.; Bianchi, L. Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis 2004, 25, 1427–1433. [Google Scholar]
- Selvendiran, K.; Koga, H.; Ueno, T.; Yoshida, T.; Maeyama, M.; Torimura, T.; Yano, H.; Kojiro, M.; Sata, M. Luteolin Promotes Degradation in Signal Transducer and Activator of Transcription 3 in Human Hepatoma Cells: An Implication for the Antitumor Potential of Flavonoids. Cancer Res 2006, 66, 4826–4834. [Google Scholar]
- Georgetti, S.R.; Casagrande, R.; Vicentini, F.T.M.C.; Verri, W.A., Jr; Fonseca, M.J.V. Evaluation of the antioxidant activity of soybean extract by different in vitro methods and investigation of this activity after its incorporation in topical formulations. Eur. J. Pharm. Biopharm 2006, 64, 99–106. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical. Biol. Med 1999, 26, 1231–1237. [Google Scholar]
- Rex, J.H.; Alexander, B.D.; Andes, D.; Arthington-Skaggs, B.; Brown, S.D.; Chaturvedi, V.; Ghannoum, M.A.; Espinel-Ingroff, A.; Knapp, C.C.; Ostrosky-Zeichner, L.; et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed; CLSI document M27-A3; ISBN 1-56238-666-2. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2002. [Google Scholar]
- Wikler, M.A.; Hindler, J.F.; Cockerill, F.R.; Patel, J.B.; Bush, K.; Powell, M.; Dudley, M.N.; Turnidge, J.D.; Elopoulos, G.M.; Weinstein, M.P.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, 8th ed; CLSI document M07-A8; ISBN ISBN 1-56238-689-1. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Kuete, V.; Nanab, F.; Ngamenic, B.; Mbaveng, A.T.; Keumedjio, F.; Ngadjuib, B.T. Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae). J. Ethnopharmacol 2009, 124, 556–561. [Google Scholar]
- Zhang, J.D.; Xu, Z.; Cao, Y.B.; Chen, H.S.; Yan, L.; An, M.M.; Gao, P.H.; Wang, Y.; Jia, X.M.; Jiang, Y.Y. Antifungal activities and action mechanisms of compounds from Tribulus terrestris L. J. Ethnopharmacol 2006, 103, 76–84. [Google Scholar]
- Ding, W.J.; Hasegawa, T.; Peng, D.; Hosaka, H.; Seko, Y. Preliminary investigation on the cytotoxicity of tellurite to cultured HeLa cells. J. Trace. Elem. Med. Biol 2002, 16, 99–102. [Google Scholar]
- He, M.; Du, M.; Fan, M.; Bian, Z. In vitro activity of eugenol against Candida albicans biofilms. Mycopathologia 2007, 163, 137–143. [Google Scholar]







| Samples | IC50 a |
|---|---|
| Means ± SD | |
| L. spiralis leaves | 1.743 ± 0.063 b |
| quercetin | 1.140 ± 0.038 b |
| luteolin | 1.215 ± 0.031 b |
| Microorganism | L. spiralis | Luteolin | ||
|---|---|---|---|---|
| MIC a | MBC/MFC a | MIC a | MBC/MFC a | |
| C. albicans | 500 | 1000 | 125 | 500 |
| C. krusei | 500 | 1000 | 250 | 250 |
| C. parapsilosis | 250 | 250 | 125 | 125 |
| C. tropicalis | 1000 | 1000 | 250 | 250 |
| S. aureus | 1000 | 1000 | 125 | 125 |
| B. subtilis | 1000 | 1000 | 62.5 | 125 |
| E. faecalis | 500 | - | 31.25 | 125 |
| E. coli | - | - | 250 | 250 |
| P. aeruginosa | - | - | 62.5 | 250 |
| S. setubal | - | - | 250 | - |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Freitas Araújo, M.G.; Hilário, F.; Vilegas, W.; Dos Santos, L.C.; Brunetti, I.L.; Sotomayor, C.E.; Bauab, T.M. Correlation among Antioxidant, Antimicrobial, Hemolytic, and Antiproliferative Properties of Leiothrix spiralis Leaves Extract. Int. J. Mol. Sci. 2012, 13, 9260-9277. https://doi.org/10.3390/ijms13079260
De Freitas Araújo MG, Hilário F, Vilegas W, Dos Santos LC, Brunetti IL, Sotomayor CE, Bauab TM. Correlation among Antioxidant, Antimicrobial, Hemolytic, and Antiproliferative Properties of Leiothrix spiralis Leaves Extract. International Journal of Molecular Sciences. 2012; 13(7):9260-9277. https://doi.org/10.3390/ijms13079260
Chicago/Turabian StyleDe Freitas Araújo, Marcelo Gonzaga, Felipe Hilário, Wagner Vilegas, Lourdes Campaner Dos Santos, Iguatemy Lourenço Brunetti, Claudia Elena Sotomayor, and Tais Maria Bauab. 2012. "Correlation among Antioxidant, Antimicrobial, Hemolytic, and Antiproliferative Properties of Leiothrix spiralis Leaves Extract" International Journal of Molecular Sciences 13, no. 7: 9260-9277. https://doi.org/10.3390/ijms13079260





