Deciphering the Molecular Nature of Ovarian Cancer Biomarker CA125
Abstract
:1. Introduction
2. Biological Function
3. Nucleotide and Amino Acid Sequence
4. Antigenic Determinants
5. Forms and Variants
6. Oligosaccharide Moieties
7. Mass Spectrometric Identification of CA125
8. Conclusion
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Bast, R.C., Jr; Feeney, M.; Lazarus, H.; Nadler, L.M.; Colvin, R.B.; Knapp, R.C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Invest. 1981, 68, 1331–1317. [Google Scholar]
- Bast, R.C., Jr; Klug, T.L.; St John, E.; Jenison, E.; Niloff, J.M.; Lazarus, H.; Berkowitz, R.S.; Leavitt, T.; Griffiths, C.T.; Parker, L.; et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med. 1983, 309, 883–887. [Google Scholar]
- Muyldermans, M.; Cornillie, F.J.; Koninckx, P.R. CA125 and endometriosis. Hum. Reprod. Update 1995, 1, 173–187. [Google Scholar]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br. J. Obstet. Gynaecol 1990, 97, 922–929. [Google Scholar]
- Bast, R.C., Jr.; Badgwell, D.; Lu, Z.; Marquez, R.; Rosen, D.; Liu, J.; Baggerly, K.A.; Atkinson, E.N.; Skates, S.; Zhang, Z.; et al. New tumor markers: CA125 and beyond. Int. J. Gynecol. Cancer 2005, 15 Suppl 3, S274–281. [Google Scholar]
- Menon, U.; Gentry-Maharaj, A.; Hallett, R.; Ryan, A.; Burnell, M.; Sharma, A.; Lewis, S.; Davies, S.; Philpott, S.; Lopes, A.; et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: Results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol 2009, 10, 327–340. [Google Scholar]
- Kui Wong, N.; Easton, R.L.; Panico, M.; Sutton-Smith, M.; Morrison, J.C.; Lattanzio, F.A.; Morris, H.R.; Clark, G.F.; Dell, A.; Patankar, M.S. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J. Biol. Chem 2003, 278, 28619–28634. [Google Scholar]
- Patankar, M.S.; Jing, Y.; Morrison, J.C.; Belisle, J.A.; Lattanzio, F.A.; Deng, Y.; Wong, N.K.; Morris, H.R.; Dell, A.; Clark, G.F. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol. Oncol 2005, 99, 704–713. [Google Scholar]
- Belisle, J.A.; Gubbels, J.A.; Raphael, C.A.; Migneault, M.; Rancourt, C.; Connor, J.P.; Patankar, M.S. Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125). Immunology 2007, 122, 418–429. [Google Scholar]
- Zhang, J.Q.; Nicoll, G.; Jones, C.; Crocker, P.R. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J. Biol. Chem 2000, 275, 22121–22126. [Google Scholar]
- Belisle, J.A.; Horibata, S.; Jennifer, G.A.; Petrie, S.; Kapur, A.; Andre, S.; Gabius, H.J.; Rancourt, C.; Connor, J.; Paulson, J.C.; et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol. Cancer 2010, 9, 118. [Google Scholar]
- Seelenmeyer, C.; Wegehingel, S.; Lechner, J.; Nickel, W. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J. Cell Sci 2003, 116 Pt 7, 1305–1318. [Google Scholar]
- Demydenko, D.; Berest, I. Expression of galectin-1 in malignant tumors. Exp. Oncol 2009, 31, 74–79. [Google Scholar]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem 2004, 279, 9190–9198. [Google Scholar]
- Rodriguez, G.C.; Haisley, C.; Hurteau, J.; Moser, T.L.; Whitaker, R.; Bast, R.C., Jr; Stack, M.S. Regulation of invasion of epithelial ovarian cancer by transforming growth factor-beta. Gynecol. Oncol. 2001, 80, 245–253. [Google Scholar]
- O’Brien, T.J.; Tanimoto, H.; Konishi, I.; Gee, M. More than 15 years of CA 125: What is known about the antigen, its structure and its function. Int. J. Biol. Markers 1998, 13, 188–195. [Google Scholar]
- Yin, B.W.; Lloyd, K.O. Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16. J. Biol. Chem 2001, 276, 27371–27375. [Google Scholar]
- O’Brien, T.J.; Beard, J.B.; Underwood, L.J.; Dennis, R.A.; Santin, A.D.; York, L. The CA 125 gene: An extracellular superstructure dominated by repeat sequences. Tumour Biol 2001, 22, 348–366. [Google Scholar]
- McLemore, M.R.; Aouizerat, B. Introducing the MUC16 gene: Implications for prevention and early detection in epithelial ovarian cancer. Biol. Res. Nurs 2005, 6, 262–267. [Google Scholar]
- Hovig, E.; Rye, P.D.; Warren, D.J.; Nustad, K. CA 125: The end of the beginning. Tumour Biol 2001, 22, 345–347. [Google Scholar]
- O’Brien, T.J.; Beard, J.B.; Underwood, L.J.; Shigemasa, K. The CA 125 gene: A newly discovered extension of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumour Biol 2002, 23, 154–169. [Google Scholar]
- Maeda, T.; Inoue, M.; Koshiba, S.; Yabuki, T.; Aoki, M.; Nunokawa, E.; Seki, E.; Matsuda, T.; Motoda, Y.; Kobayashi, A.; et al. Solution structure of the SEA domain from the murine homologue of ovarian cancer antigen CA125 (MUC16). J. Biol. Chem 2004, 279, 13174–13182. [Google Scholar]
- Berman, Z.T.; Moore, L.J.; Knudson, K.E.; Whelan, R.J. Synthesis and structural characterization of the peptide epitope of the ovarian cancer biomarker CA125 (MUC16). Tumour Biol 2010, 31, 495–502. [Google Scholar]
- Bork, P.; Patthy, L. The SEA module: A new extracellular domain associated with O-glycosylation. Protein Sci 1995, 4, 1421–1425. [Google Scholar]
- Julenius, K.; Molgaard, A.; Gupta, R.; Brunak, S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 2005, 15, 153–164. [Google Scholar]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. UniProt: The Universal Protein knowledgebase. Nucleic Acids Res 2004, 32(Database issue), D115–119. [Google Scholar]
- Konishi, I.; Fendrick, J.L.; Parmley, T.H.; Quirk, J.G., Jr; O’Brien, T.J. Epidermal growth factor enhances secretion of the ovarian tumor-associated cancer antigen CA125 from the human amnion WISH cell line. J. Soc. Gynecol. Investig. 1994, 1, 89–96. [Google Scholar]
- Lloyd, K.O.; Yin, B.W. Synthesis and secretion of the ovarian cancer antigen CA 125 by the human cancer cell line NIH:OVCAR-3. Tumour Biol 2001, 22, 77–82. [Google Scholar]
- Parry, S.; Silverman, H.S.; McDermott, K.; Willis, A.; Hollingsworth, M.A.; Harris, A. Identification of MUC1 proteolytic cleavage sites in vivo. Biochem. Biophys. Res. Commun 2001, 283, 715–720. [Google Scholar]
- Palmai-Pallag, T.; Khodabukus, N.; Kinarsky, L.; Leir, S.H.; Sherman, S.; Hollingsworth, M.A.; Harris, A. The role of the SEA (sea urchin sperm protein, enterokinase and agrin) module in cleavage of membrane-tethered mucins. FEBS J 2005, 272, 2901–2911. [Google Scholar]
- Nustad, K.; Bast, R.C., Jr; Brien, T.J.; Nilsson, O.; Seguin, P.; Suresh, M.R.; Saga, T.; Nozawa, S.; Bormer, O.P.; de Bruijn, H.W.; et al. Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: First report from the ISOBM TD-1 workshop. International Society for Oncodevelopmental Biology and Medicine. Tumour Biol. 1996, 17, 196–219. [Google Scholar]
- Nustad, K.; Lebedin, Y.; Lloyd, K.O.; Shigemasa, K.; de Bruijn, H.W.; Jansson, B.; Nilsson, O.; Olsen, K.H.; O’Brien, T.J. Epitopes on CA 125 from cervical mucus and ascites fluid and characterization of six new antibodies. Third report from the ISOBM TD-1 workshop. Tumour Biol 2002, 23, 303–314. [Google Scholar]
- Warren, D.J.; Nustad, K.; Beard, J.B.; O’Brien, T.J. Expression and epitope characterization of a recombinant CA 125 repeat: Fourth report from the ISOBM TD-1 workshop. Tumour Biol 2009, 30, 51–60. [Google Scholar]
- Yin, B.W.; Dnistrian, A.; Lloyd, K.O. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int. J. Cancer 2002, 98, 737–740. [Google Scholar]
- Davis, H.M.; Zurawski, V.R., Jr.; Bast, R.C., Jr.; Klug, T.L. Characterization of the CA 125 antigen associated with human epithelial ovarian carcinomas. Cancer Res 1986, 46 12 Pt 1, 6143–6148. [Google Scholar]
- Halila, H. Detection of ovarian cancer marker CA 125 in human seminal plasma. Tumour Biol 1985, 6, 207–212. [Google Scholar]
- De los Frailes, M.T.; Stark, S.; Jaeger, W.; Hoerauf, A.; Wildt, L. Purification and characterization of the CA 125 tumor-associated antigen from human ascites. Tumour Biol 1993, 14, 18–29. [Google Scholar]
- Bouanene, H.; Miled, A. Conflicting views on the molecular structure of the cancer antigen CA125/MUC16. Dis. Markers 2010, 28, 385–394. [Google Scholar]
- Grimwood, J.; Gordon, L.A.; Olsen, A.; Terry, A.; Schmutz, J.; Lamerdin, J.; Hellsten, U.; Goodstein, D.; Couronne, O.; Tran-Gyamfi, M.; et al. The DNA sequence and biology of human chromosome 19. Nature 2004, 428, 529–535. [Google Scholar]
- Lloyd, K.O.; Yin, B.W.; Kudryashov, V. Isolation and characterization of ovarian cancer antigen CA 125 using a new monoclonal antibody (VK-8): Identification as a mucin-type molecule. Int. J. Cancer 1997, 71, 842–850. [Google Scholar]
- Weiland, F.; Fritz, K.; Oehler, M.K.; Hoffmann, P. Methods for identification of CA125 from ovarian cancer ascites by high resolution mass spectrometry. Int. J. Mol. Sci 2012, 13, 9942–9958. [Google Scholar]
- Tian, E.; Ten Hagen, K.G. Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj. J 2009, 26, 325–334. [Google Scholar]
- Hanisch, F.G.; Uhlenbruck, G.; Dienst, C.; Stottrop, M.; Hippauf, E. Ca 125 and Ca 19-9: Two cancer-associated sialylsaccharide antigens on a mucus glycoprotein from human milk. Eur. J. Biochem 1985, 149, 323–330. [Google Scholar]
- Nagata, A.; Hirota, N.; Sakai, T.; Fujimoto, M.; Komoda, T. Molecular nature and possible presence of a membranous glycan-phosphatidylinositol anchor of CA125 antigen. Tumour Biol 1991, 12, 279–286. [Google Scholar]
- Easton, R.L.; Patankar, M.S.; Clark, G.F.; Morris, H.R.; Dell, A. Pregnancy-associated changes in the glycosylation of tamm-horsfall glycoprotein. Expression of sialyl Lewis(x) sequences on core 2 type O-glycans derived from uromodulin. J. Biol. Chem 2000, 275, 21928–21938. [Google Scholar]
- Pastan, I.; Lovelace, E.T.; Gallo, M.G.; Rutherford, A.V.; Magnani, J.L.; Willingham, M.C. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res 1991, 51, 3781–3787. [Google Scholar]
- Yin, B.W.; Finstad, C.L.; Kitamura, K.; Federici, M.G.; Welshinger, M.; Kudryashov, V.; Hoskins, W.J.; Welt, S.; Lloyd, K.O. Serological and immunochemical analysis of Lewis y (Ley) blood group antigen expression in epithelial ovarian cancer. Int. J. Cancer 1996, 65, 406–412. [Google Scholar]
- Jankovic, M.M.; Milutinovic, B.S. Glycoforms of CA125 antigen as a possible cancer marker. Cancer Biomark 2008, 4, 35–42. [Google Scholar]
- Mitic, N.; Milutinovic, B.; Jankovic, M. Assessment of sialic acid diversity in cancer- and non-cancer related CA125 antigen using sialic acid-binding Ig-like lectins (Siglecs). Dis. Markers 2012, 32, 187–194. [Google Scholar]
- Bouanene, H.; Saibi, W.; Mokni, M.; Sriha, B.; Ben Fatma, L.; Ben Limem, H.; Ben Ahmed, S.; Gargouri, A.; Miled, A. Biochemical and morphological differences between CA125 isolated from healthy women and patients with epithelial ovarian cancer from Tunisian population. Pathol. Oncol. Res 2012, 18, 325–330. [Google Scholar]
- Jankovic, M.M.; Tapuskovic, B.S. Molecular forms and microheterogeneity of the oligosaccharide chains of pregnancy-associated CA125 antigen. Hum. Reprod 2005, 20, 2632–2638. [Google Scholar]
- Milutinovic, B.; Jankovic, M. Analysis of the protein and glycan partsof CA125 Antigen from human amniotic fluid. Arch. Biol. Sci. Belgrade 2007, 59, 97–103. [Google Scholar]
- De Souza, G.A.; Godoy, L.M.; Mann, M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol 2006, 7, R72. [Google Scholar]
- Andersch-Bjorkman, Y.; Thomsson, K.A.; Holmen Larsson, J.M.; Ekerhovd, E.; Hansson, G.C. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol. Cell. Proteomics 2007, 6, 708–716. [Google Scholar]
- Davies, J.R.; Kirkham, S.; Svitacheva, N.; Thornton, D.J.; Carlstedt, I. MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. Int. J. Biochem. Cell Biol 2007, 39, 1943–1954. [Google Scholar]
- Taylor, C.F.; Paton, N.W.; Lilley, K.S.; Binz, P.A.; Julian, R.K., Jr; Jones, A.R.; Zhu, W.; Apweiler, R.; Aebersold, R.; Deutsch, E.W.; et al. The minimum information about a proteomics experiment (MIAPE). Nat. Biotechnol. 2007, 25, 887–893. [Google Scholar]
- Taylor, C.F.; Binz, P.A.; Aebersold, R.; Affolter, M.; Barkovich, R.; Deutsch, E.W.; Horn, D.M.; Huhmer, A.; Kussmann, M.; Lilley, K.; et al. Guidelines for reporting the use of mass spectrometry in proteomics. Nat. Biotechnol 2008, 26, 860–861. [Google Scholar]
- Binz, P.A.; Barkovich, R.; Beavis, R.C.; Creasy, D.; Horn, D.M.; Julian, R.K., Jr; Seymour, S.L.; Taylor, C.F.; Vandenbrouck, Y. Guidelines for reporting the use of mass spectrometry informatics in proteomics. Nat. Biotechnol. 2008, 26, 862. [Google Scholar]
- States, D.J.; Omenn, G.S.; Blackwell, T.W.; Fermin, D.; Eng, J.; Speicher, D.W.; Hanash, S.M. Challenges in deriving high-confidence protein identifications from data gathered by a HUPO plasma proteome collaborative study. Nat. Biotechnol 2006, 24, 333–338. [Google Scholar]
- Schober, Y.; Schramm, T.; Spengler, B.; Rompp, A. Protein identification by accurate mass matrix-assisted laser desorption/ionization imaging of tryptic peptides. Rapid. Commun. Mass Spectrom 2011, 25, 2475–2483. [Google Scholar]
- Ji, Q.C.; Rodila, R.; Gage, E.M.; El-Shourbagy, T.A. A strategy of plasma protein quantitation by selective reaction monitoring of an intact protein. Anal. Chem 2003, 75, 7008–7014. [Google Scholar]
- Chen, Y.; Clark, S.; Wong, T.; Dennis, M.S.; Luis, E.; Zhong, F.; Bheddah, S.; Koeppen, H.; Gogineni, A.; Ross, S.; et al. Armed antibodies targeting the mucin repeats of the ovarian cancer antigen, MUC16, are highly efficacious in animal tumor models. Cancer Res 2007, 67, 4924–4932. [Google Scholar]
- Reinartz, S.; Kohler, S.; Schlebusch, H.; Krista, K.; Giffels, P.; Renke, K.; Huober, J.; Mobus, V.; Kreienberg, R.; DuBois, A.; et al. Vaccination of patients with advanced ovarian carcinoma with the anti-idiotype ACA125: Immunological response and survival (phase Ib/II). Clin. Cancer Res 2004, 10, 1580–1587. [Google Scholar]
- Leffers, N.; Daemen, T.; Helfrich, W.; Boezen, H.M.; Cohlen, B.J.; Melief, K.; Nijman, H.W. Antigen-specific active immunotherapy for ovarian cancer. Cochrane Database Syst. Rev 2010, 1, CD007287. [Google Scholar]
- Oei, A.L.; Sweep, F.C.; Thomas, C.M.; Boerman, O.C.; Massuger, L.F. The use of monoclonal antibodies for the treatment of epithelial ovarian cancer (review). Int. J. Oncol 2008, 32, 1145–1157. [Google Scholar]
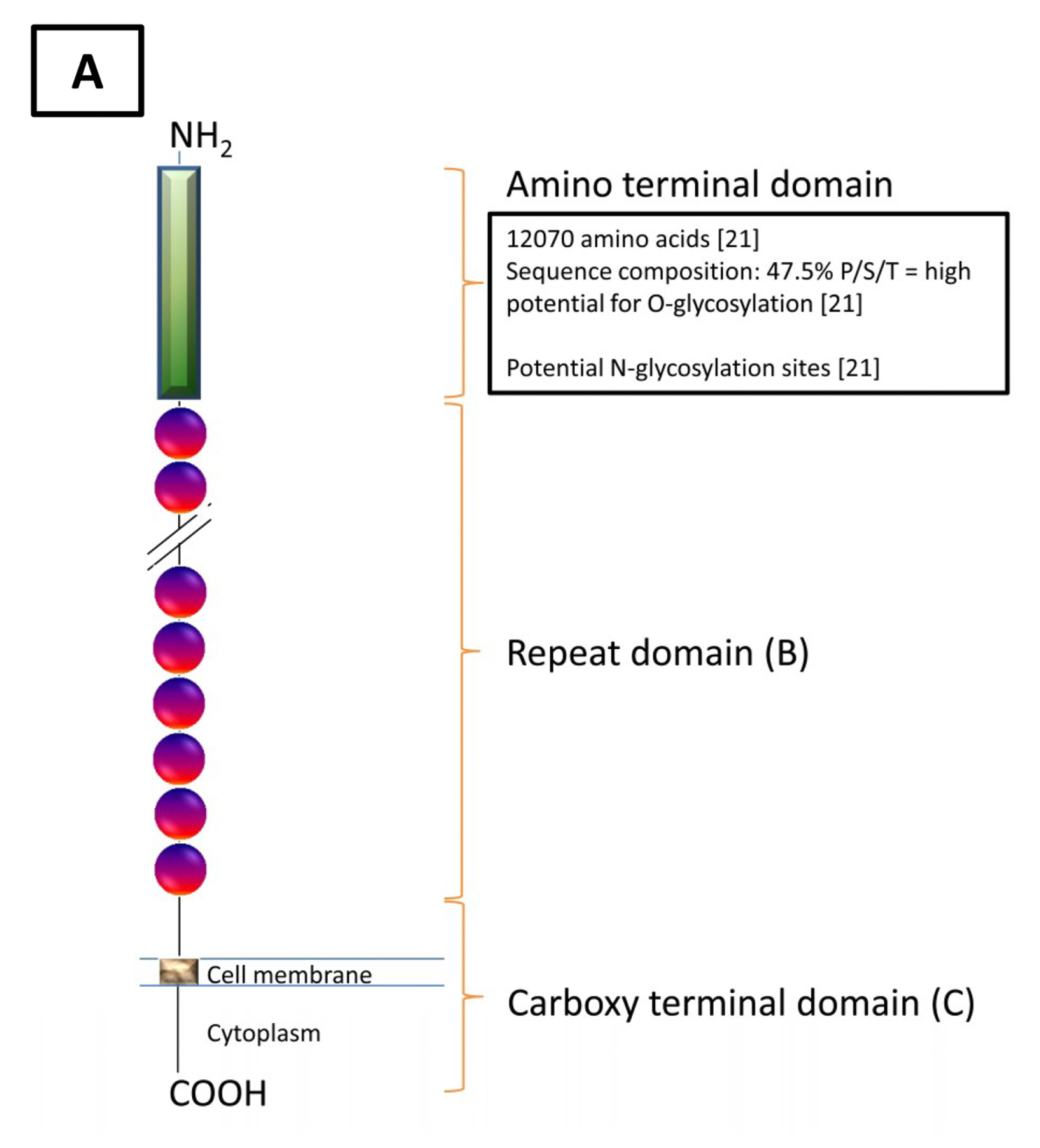

| Domain | Carboxy Domain | Repeat Domain | Amino Domain |
|---|---|---|---|
| Features | |||
| Exons | 9 | 5 | 9 |
| Nucleotides (bp) | 14,000 | Single: 1900 Cumulative: Unknown | 50,950 |
| Amino Acids (aa) | 284 | Single: 156 Cumulative: >9360 | 12,068 |
| Molecular Characteristics | Stop CodonPoly A Signal Potential Transmembrane Region (aa 230–252) Potential Cytoplasmic Region (aa 256–260) Potential Proteolytic Cleavage Sites (aa 171–181 [18] and aa 35/36 [22]) | SEA domain Cysteine loop (aa 59,79) [23] Conserved Methionine (aa 24) Serine/Threonine/Proline Rich | Serine/Threonine Rich(Abundant O-glycosylation Potential, N-glycosylation Potential) |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Weiland, F.; Martin, K.; Oehler, M.K.; Hoffmann, P. Deciphering the Molecular Nature of Ovarian Cancer Biomarker CA125. Int. J. Mol. Sci. 2012, 13, 10568-10582. https://doi.org/10.3390/ijms130810568
Weiland F, Martin K, Oehler MK, Hoffmann P. Deciphering the Molecular Nature of Ovarian Cancer Biomarker CA125. International Journal of Molecular Sciences. 2012; 13(8):10568-10582. https://doi.org/10.3390/ijms130810568
Chicago/Turabian StyleWeiland, Florian, Karina Martin, Martin K. Oehler, and Peter Hoffmann. 2012. "Deciphering the Molecular Nature of Ovarian Cancer Biomarker CA125" International Journal of Molecular Sciences 13, no. 8: 10568-10582. https://doi.org/10.3390/ijms130810568
APA StyleWeiland, F., Martin, K., Oehler, M. K., & Hoffmann, P. (2012). Deciphering the Molecular Nature of Ovarian Cancer Biomarker CA125. International Journal of Molecular Sciences, 13(8), 10568-10582. https://doi.org/10.3390/ijms130810568





