Constituents from Vigna vexillata and Their Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification and Characterization
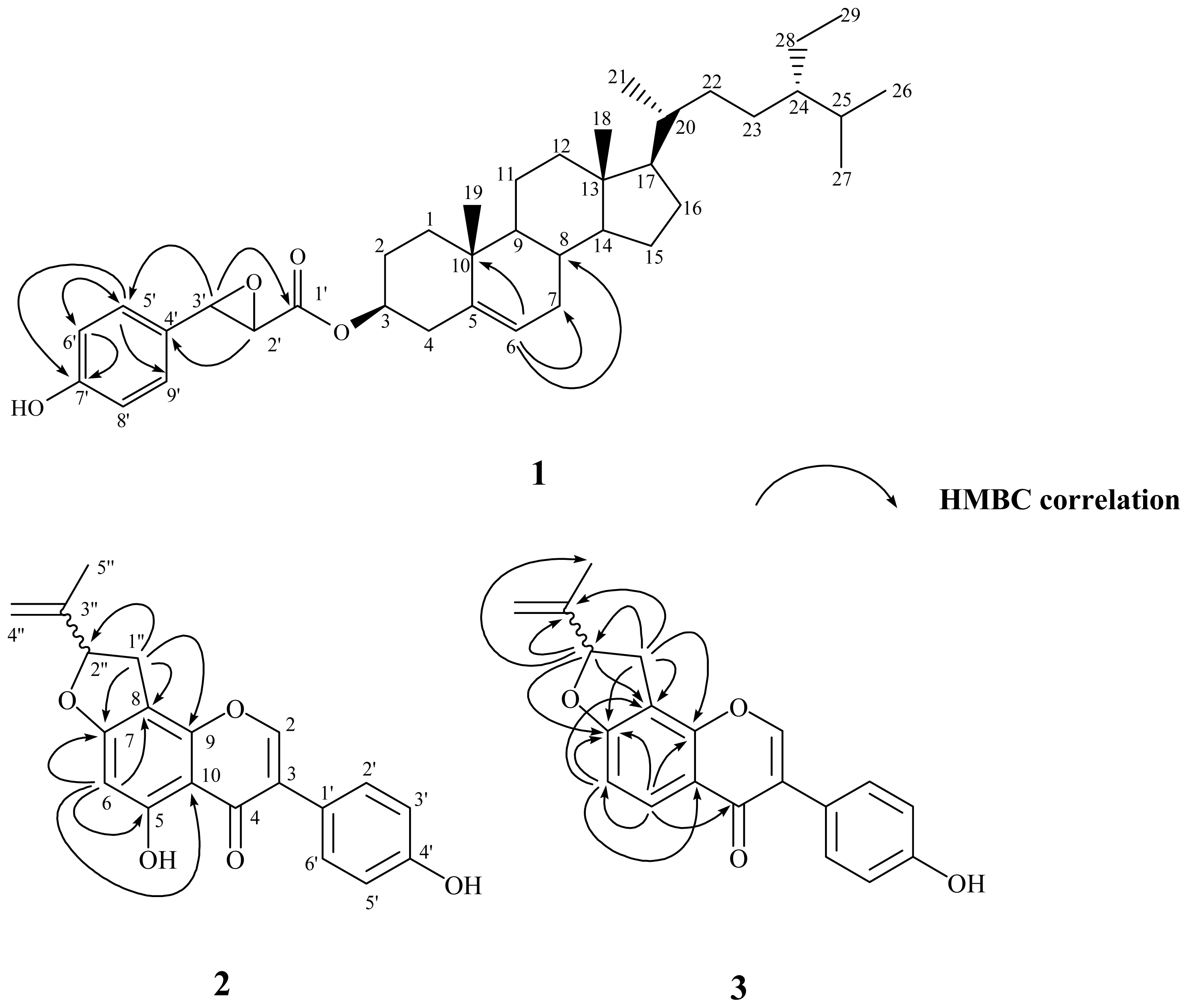
2.2. Structural Elucidation of New Compounds 1–3
2.3. Anti-Inflammatory Activity
3. Experimental Section
3.1. General
3.2. Plant Materials
3.3. Extraction and Isolation
3.3.1. Vignasterol A (1)
3.3.2. Vigvexin A (2)
3.3.3. Vigvexin B (3)
3.4. Anti-Inflammatory Activity
3.4.1. Preparation of Human Neutrophils
3.4.2. Measurement of Superoxide Anion Generation
3.4.3. Measurement of Elastase Release
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors have no conflict of interest to report.
References
- Shu, L.; Cheung, K.L.; Khor, T.O.; Chen, C.; Kong, A.N. Phytochemicals: Cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev 2010, 29, 483–502. [Google Scholar]
- Johnson, S.M.; Wang, X.; Evers, B.M. Triptolide inhibits proliferation and migration of colon cancer cells by inhibition of cell cycle regulators and cytokine receptors. J. Surg. Res 2011, 168, 197–205. [Google Scholar]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep 2000, 17, 215–234. [Google Scholar]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res 2003, 23, 363–398. [Google Scholar]
- Bar-Sela, G.; Epelbaum, R.; Schaffer, M. Curcumin as an anti-cancer agent: Review of the gap between basic and clinical applications. Curr. Med. Chem 2010, 17, 190–197. [Google Scholar]
- Na, H.K.; Kim, E.H.; Jung, J.H.; Lee, H.H.; Hyun, J.W.; Surh, Y.J. (−)-Epigallocatechin gallate induces Nrf 2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch. Biochem. Biophys. 2008, 476, 171–177. [Google Scholar]
- Swami, S.; Krishnan, A.V.; Moreno, J.; Bhattacharyya, R.S.; Gardner, C.; Brooks, J.D.; Peehl, D.M.; Feldman, D. Inhibition of prostaglandin synthesis and actions by genistein in human prostate cancer cells and by soy isoflavones in prostate cancer patients. Int. J. Cancer 2009, 124, 2050–2059. [Google Scholar]
- Zhou, Y.Y.; Luo, S.H.; Yi, T.S.; Li, C.H.; Luo, Q.; Hua, J.; Liu, Y.; Li, S.H. Secondary metabolites from Glycine soja and their growth inhibitory effect against Spodoptera litura. J. Agric. Food Chem 2011, 59, 6004–6010. [Google Scholar]
- Gunjatkar, N.; Vartak, V.D. Enumeration of wild legumes from Pune district, Maharashtra State. J. Econ. Tax. Bot 1982, 3, 1–9. [Google Scholar]
- Siddhuraju, P.; Vijayakumari, K.; Janardhanan, K. Chemical analysis and nutritional assessment of the less known pulses, Vigna aconitifolia (Jacq.) Marechal and Vigna vexillata (L.) A. Rich. Plant Food Hum. Nutr 1994, 45, 103–111. [Google Scholar]
- Huang, T.C.; Ohashi, H. Flora of Taiwan, 2nd ed; Editorial Committee of Flora of Taiwan: Taipei, Taiwan, 1993; Volume 3, p. 393. [Google Scholar]
- Itoh, T.; Kita, N.; Kurokawa, Y.; Kobayashi, M.; Horio, F.; Furuichi, Y. Suppressive effect of a hot water extract of adzuki beans (Vigna angularis) on hyperglycemia after sucrose loading in mice and diabetic rats. Biosci. Biotechnol. Biochem 2004, 68, 2421–2426. [Google Scholar]
- Itoh, T.; Kobayashi, M.; Horio, F.; Furuichi, Y. Hypoglycemic effect of hot-water extract of adzuki beans (Vigna angularis) in spontaneously diabetic KK-Ay mice. Nutrition 2009, 25, 134–141. [Google Scholar]
- Mukai, Y.; Sato, S. Polyphenol-Containing adzuki bean (Vigna angularis) extract attenuates blood pressure elevation and modulates nitric oxide synthase and caveolin-1 expressions in rats with hypertension. Nutr. Metab. Cardiovasc. Dis 2009, 19, 491–497. [Google Scholar]
- Mukai, Y.; Sato, S. Polyphenol-containing azuki bean (Vigna angularis) seed coats attenuate vascular oxidative stress and inflammation in spontaneously hypertensive rats. J. Nutr. Biochem 2011, 22, 16–21. [Google Scholar]
- Itoh, T.; Furuichi, Y. Lowering serum cholesterol level by feeding a 40% ethanol-eluted fraction from HP-20 resin treated with hot water extract of adzuki beans (Vigna angularis) to rats fed a high-fat cholesterol diet. Nutrition 2009, 25, 318–321. [Google Scholar]
- Ariga, T.; Koshiyama, I.; Fukushima, D. Antioxidative properties of procyanidins B-1 and B-3 from adzuki beans in aqueous systems. Agric. Biol. Chem 1988, 52, 2717–2722. [Google Scholar]
- Doblado, R.; Zielinski, H.; Piskula, M.; Kozlowska, H.; Muñoz, R.; Frías, J.; Vidal-Valverde, C. Effect of processing on the antioxidant vitamins and antioxidant capacity of Vigna sinensis var. carilla. J. Agric. Food Chem 2005, 53, 1215–1222. [Google Scholar]
- Hori, Y.; Sato, S.; Hatai, A. Antibacterial activity of plant extracts from adzuki beans (Vigna angularis) in vitro. Phytother. Res 2006, 20, 162–164. [Google Scholar]
- Franco, O.L.; Murad, A.M.; Leite, J.R.; Mendes, P.A.M.; Prates, M.V.; Bloch, C., Jr. Identification of a cowpea γ-thionin with bactericidal activity. FEBS J. 2006, 273, 3489–3497. [Google Scholar]
- Itoh, T.; Itoh, Y.; Mizutani, M.; Fujishiro, K.; Furuichi, Y.; Komiya, T.; Hibasami, H. Hot-water extracts from adzuki beans (Vigna angularis) suppress not only proliferation of KATO III cells in culture but also benzo(a)pyrene-induced tumorigenesis in mouse forestomatch. J. Nutr. Sci. Vitaminol 2004, 50, 295–299. [Google Scholar]
- Itoh, T.; Furuichi, Y. Hot-Water extracts from adzuki beans (Vigna angularis) stimulated not only melanogenesis in cultured mouse B16 melanoma cells but also pigmentation of hair color in C3H mice. Biosci. Biotechnol. Biochem 2005, 69, 873–882. [Google Scholar]
- Joanitii, G.A.; Azevedo, R.B.; Freitas, S.M. Apoptosis and lysosome membrane permeabilization induction on breast cancer cells by an anticarcinogenic Bowman-Birk protease inhibitor from Vigna unguiculata seeds. Cancer Lett 2010, 29, 73–81. [Google Scholar]
- Ambrus, G.; Ilköy, E.; Jekkel, A.; Horváth, G.; Böcskei, Z. Microbial transformation of β-sitosterol and stigmasterol into 26-oxygenated derivatives. Steroids 1995, 60, 621–625. [Google Scholar]
- Kitajima, J.; Kimizuka, K.; Tanaka, Y. New sterols and triterpenoids of Ficus pumila fruit. Chem. Pharm. Bull 1998, 46, 1408–1411. [Google Scholar]
- Kuo, Y.H.; Li, Y.C. Constituents of the bark of Ficus microcarpa L.f. J. Chin. Chem. Soc 1997, 44, 321–325. [Google Scholar]
- Lin, W.Y.; Yen, M.H.; Teng, C.M.; Tsai, I.L.; Chen, I.S. Cerebrosides from the rhizomes of Gynura japonica. J. Chin. Chem. Soc 2004, 51, 1429–1434. [Google Scholar]
- Yasukawa, K.; Akihisa, T.; Kimura, Y.; Tamura, T.; Takido, M. Inhibitory effect of cycloartenol ferulate, a component of rice bran, on tumor promotion in two-stage carcinogenesis in mouse skin. Biol. Pharm. Bull 1998, 21, 1072–1076. [Google Scholar]
- Kontiza, I.; Abatis, D.; Malakate, K.; Vagias, C.; Roussis, V. 3-Keto steroids from the marine organisms Dendrophyllia cornigera and Cymodocea nodosa. Steroids 2006, 71, 177–181. [Google Scholar]
- Chen, C.Y.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Cheritamine, a new N-fatty acyl tryptamine and other constituents from the stem of Annona cherimola. J. Chin. Chem. Soc 1999, 46, 77–86. [Google Scholar]
- Garcez, W.S.; Martins, D.; Garcez, F.R.; Marques, M.R.; Pereira, A.A.; Oliveira, L.A.; Rondon, J.N.; Peruca, A.D. Effect of spores of saprophytic fungi on phytoalexin accumulation in seeds of frog-eye leaf spot and stem canker-resistant and -susceptible soybean (Glycine max L.) cultivars. J. Agric. Food Chem 2000, 48, 3662–3665. [Google Scholar]
- Serra, S.; Barakat, A.; Fuganti, C. Chemoenzymatic resolution of cis- and trans-3,6-dihydroxy-α-ionone. Synthesis of the enantiomeric forms of dehydrovomifoliol and 8,9-dehydrotheaspirone. Tetrahedron Asymmetry 2007, 18, 2573–2580. [Google Scholar]
- Jones, J.B.; Baskevitch, N. Steroids and steroidases XX (1). Aggregation in aqueous solution of steroids with stigmastane type C-17 side chains and its influence on their enzymic transformations. Steroids 1973, 22, 525–538. [Google Scholar]
- Hosny, M.; Rosazza, J.P.N. Microbial hydroxylation and methylation of genistein by Streptomycetes. J. Nat. Prod 1999, 62, 1609–1612. [Google Scholar]
- Lin, Y.L.; Tsai, W.J.; Chen, I.S.; Kuo, Y.H. Chemical constituents from Mucuna membranacea. J. Chin. Chem. Soc 1998, 45, 213–217. [Google Scholar]
- Yang, S.W.; Cordell, G.A. Metabolism studies of indole derivatives using a staurosporine producer, Streptomyces staurosporeus. J. Nat. Prod 1997, 60, 44–48. [Google Scholar]
- Smith, T.R.; Clark, A.J.; Clarkson, G.J.; Taylor, P.C.; Marsh, A. Concise enantioselective synthesis of abscisic acid and a new analogue. Org. Biomol. Chem 2006, 4, 4186–4192. [Google Scholar]
- Hsieh, T.J.; Chang, F.R.; Wu, Y.C. The constituents of Cananga odorata. J. Chin. Chem. Soc 1999, 46, 607–611. [Google Scholar]
- Faizi, S.; Ali, M.; Saleem, R.; Irfanullah; Bibi, S. Complete 1H and 13C NMR assignments of stigma-5-en-3-O-β-glucoside and its acetyl derivative. Magn. Reson. Chem. 2001, 39, 399–405. [Google Scholar]
- Kundo, M. The nuclear magnetic resonance study of several O-disubstituted benzenes. Bull. Chem. Soc. Jpn 1972, 45, 2790–2793. [Google Scholar]
- Zhong, X.N.; Otsuka, H.; Ide, T.; Hirata, E.; Takeda, Y. Hydroquinone diglycoside acyl esters from the leaves of Myrsine seguinii. Phytochemistry 1999, 52, 923–927. [Google Scholar]
- Skouroumounis, G.K.; Sefton, M.A. Acid-catalyzed hydrolysis of alcohols and their β-d-glucopyranosides. J. Agric. Food Chem 2000, 48, 2033–2039. [Google Scholar]
- Xu, Q.M.; Liu, Y.L.; Li, X.R.; Feng, Y.L.; Yang, S.L. Two new phenylglycol derivatives isolated from Syringa reticulata var. mandshurica and their antifungal activities. Chem. Pharm. Bull 2009, 57, 863–866. [Google Scholar]
- Chiang, Y.M.; Liu, H.K.; Lo, J.M.; Chien, S.C.; Chan, Y.F.; Lee, T.H.; Su, J.K.; Kuo, Y.H. Cytotoxic constituents of leaves of Calocedrus formosana. J. Chin. Chem. Soc 2003, 50, 161–166. [Google Scholar]
- Yu, Q.; Otsuka, H.; Hirata, E.; Shinzato, T.; Takeda, Y. Turpinionosides A–E: Megastigmane glucosides from leaves of Turpinia ternata Nakai. Chem. Pharm. Bull 2002, 50, 640–644. [Google Scholar]
- Masamune, T.; Anetai, M.; Fukuzawa, A.; Takasugi, M.; Matsue, H.; Kobayashi, K.; Ueno, S.; Katsui, N. Glycinoeclepins, natural hatching stimuli for the soybean cyst nematode, Heterodera glycines. I. Isolation. Bull. Chem. Soc. Jpn 1987, 60, 981–999. [Google Scholar]
- Ito, N.; Etoh, T.; Hagiwara, H.; Kato, M. Novel synthesis of degradation products of carotenoids, megastigmatrienone analogues and blumenol-A. J. Chem. Soc. Perkin Trans 1997, 1571–1579. [Google Scholar]
- Ling, T.J.; Ling, W.W.; Chen, Y.J.; Wan, X.C.; Xia, T.; Du, X.F.; Zhang, Z.Z. Antiseptic activity and phenolic constituents of the aerial parts of Vitex negundo var. cannabifolia. Molecules 2010, 15, 8469–8477. [Google Scholar]
- Weis, M.; Lim, E.K.; Bruce, N.; Bowles, D. Regioselective glucosylation of aromatic compounds: Screening of a recombinant glycosyltransferase library to identify biocatalysts. Angew. Chem. Int. Ed 2006, 45, 3534–3538. [Google Scholar]
- Wang, P.H.; Lee, S.S. Polar chemical constituents from Phoebe formosana. J. Chin. Chem. Soc 1999, 46, 215–219. [Google Scholar]
- Hua, Y.; Wang, H.Q. Chemical components of Anaphalis sinica Hance. J. Chin. Chem. Soc 2004, 51, 409–415. [Google Scholar]
- Srivastava, A.; Shukla, Y.N. Aryl esters and a coumarin from Aygyreia speciosa. Indian J. Chem. Sect 1998, 37B, 192–194. [Google Scholar]
- Scott, A.I. Interpretation Ultraviolet Spectra of Natural Products, 2nd ed; Pergamon Press: New York, NY, USA; p. 1964.
- Malech, H.L.; Gallin, J.I. Current concepts: Immunology neutrophils in human diseases. N. Engl. J. Med 1987, 317, 687–694. [Google Scholar]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Invest 2000, 80, 617–653. [Google Scholar]
- Okajima, K.; Harada, N.; Uchiba, M. Ranitidine reduces ischemia/reperfusion-induced liver injury in rats by inhibiting neutrophil activation. J. Pharmacol. Exp. Ther 2002, 301, 1157–1165. [Google Scholar]
- Ennis, M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep 2003, 3, 159–165. [Google Scholar]
- Vinten-Johansen, J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion Injury. Cardiovasc. Res 2004, 61, 481–497. [Google Scholar]
- Hwang, T.L.; Li, G.L.; Lan, Y.H.; Chia, Y.C.; Shieh, P.W.; Wu, Y.H.; Wu, Y.C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med 2009, 46, 520–528. [Google Scholar]
- Yang, M.L.; Kuo, P.C.; Hwang, T.L.; Chiou, W.F.; Qian, K.; Lai, C.Y.; Lee, K.H.; Wu, T.S. Synthesis, in vitro anti-inflammatory and cytotoxic evaluation, and mechanism of action studies of 1-benzoyl-β-carboline and 1-benzoyl-3-carboxy-β-carboline derivatives. Bioorg. Med. Chem 2011, 19, 1674–1682. [Google Scholar]
| Samples | Inhibition Percentage (%) a | |
|---|---|---|
| Superoxide Anion Generation | Elastase Release | |
| methanol extract | 22.06 ± 5.66 * | 19.35 ± 1.52 *** |
| chloroform fraction | 57.65 ± 3.69 *** | 67.27 ± 3.53 *** |
| water fraction | 11.08 ± 5.19 | 11.00 ± 3.37 * |
| Compounds | IC50 (μM) a or (Inh %) b | |
|---|---|---|
| Superoxide Anion Generation | Elastase Release | |
| 1 | (12.58 ± 0.82) *** | 8.93 ± 1.64 |
| 2 | (40.57 ± 4.06) *** | (17.27 ± 4.19) * |
| 3 | 4.05 ± 0.66 | (12.62 ± 7.17) |
| 9 | (6.13 ± 3.26) | (−21.93 ± 1.80) *** |
| 10 | (2.27 ± 2.70) | (−10.96 ± 5.47) |
| 13 | (15.45 ± 1.17) *** | (11.59 ± 4.53) |
| 19 | 1.30 ± 0.27 | (42.15 ± 2.88) *** |
| 20 | (19.26 ± 5.37) * | (11.39 ± 4.98) |
| 22 | 5.87 ± 0.50 | (19.37 ± 4.16) ** |
| 23 | 3.13 ± 0.27 | 4.29 ± 0.49 |
| 25 | 2.66 ± 0.85 | 2.71 ± 0.25 |
| 27 | (−1.35 ± 3.50) | (16.39 ± 2.85) ** |
| 30 | (37.34 ± 3.26) *** | (7.68 ± 5.60) |
| 31 | (20.28 ± 4.96) * | (20.11 ± 2.84) ** |
| 34 | (26.38 ± 6.94) * | (31.49 ± 5.00) ** |
| 35 | (26.81 ± 6.19) * | (24.43 ± 4.42) ** |
| 36 | (17.15 ± 3.77) * | (17.88 ± 1.56) *** |
| 37 | (8.97 ± 2.64) * | (4.34 ± 0.58) ** |
| 38 | (−0.88 ± 2.98) | (−1.04 ± 7.31) |
| 39 | (12.45 ± 5.61) | (−1.30 ± 3.61) |
| 40 | 4.47 ± 0.76 | 5.51 ± 1.07 |
| LY294002 c | 1.38 ± 0.22 | 1.95 ± 0.35 |
| DPI d | 0.93 ± 0.52 | – |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leu, Y.-L.; Hwang, T.-L.; Kuo, P.-C.; Liou, K.-P.; Huang, B.-S.; Chen, G.-F. Constituents from Vigna vexillata and Their Anti-Inflammatory Activity. Int. J. Mol. Sci. 2012, 13, 9754-9768. https://doi.org/10.3390/ijms13089754
Leu Y-L, Hwang T-L, Kuo P-C, Liou K-P, Huang B-S, Chen G-F. Constituents from Vigna vexillata and Their Anti-Inflammatory Activity. International Journal of Molecular Sciences. 2012; 13(8):9754-9768. https://doi.org/10.3390/ijms13089754
Chicago/Turabian StyleLeu, Yann-Lii, Tsong-Long Hwang, Ping-Chung Kuo, Kun-Pei Liou, Bow-Shin Huang, and Guo-Feng Chen. 2012. "Constituents from Vigna vexillata and Their Anti-Inflammatory Activity" International Journal of Molecular Sciences 13, no. 8: 9754-9768. https://doi.org/10.3390/ijms13089754





