Semisynthesis and Antifeedant Activity of New Derivatives of a Dihydro-β-Agarofuran from Parnassia wightiana
Abstract
:1. Introduction
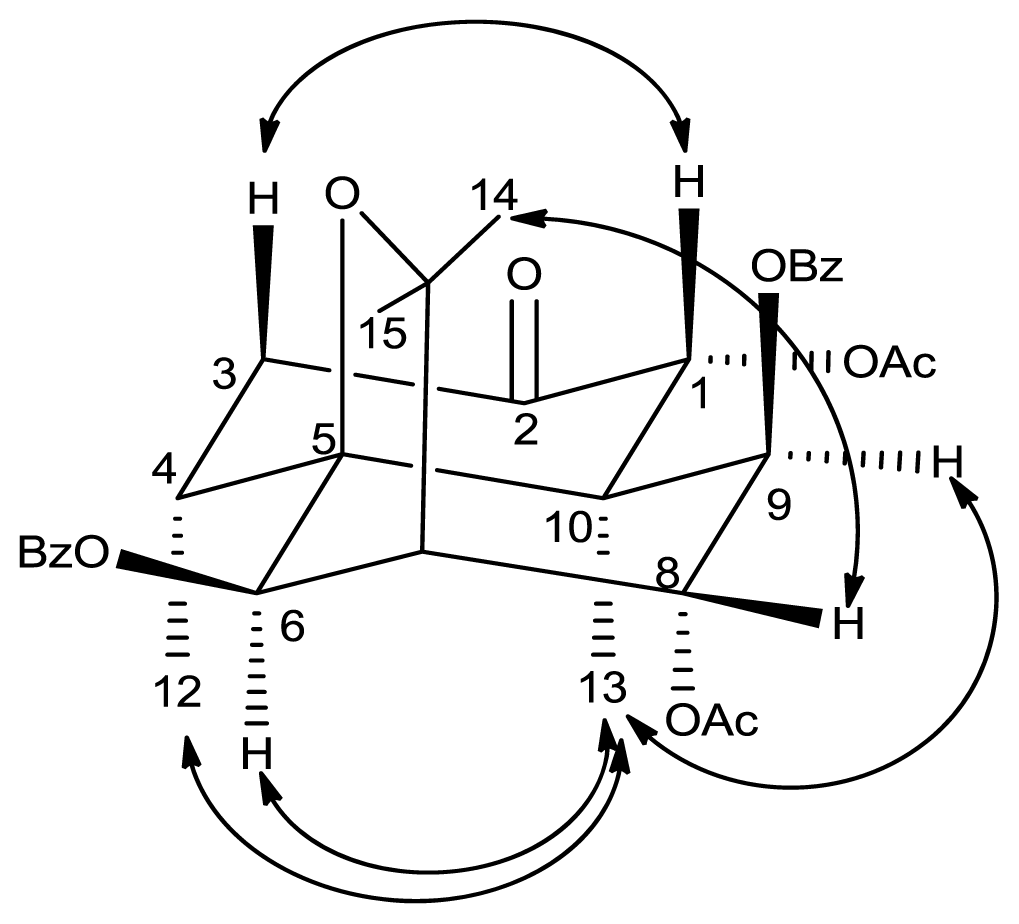
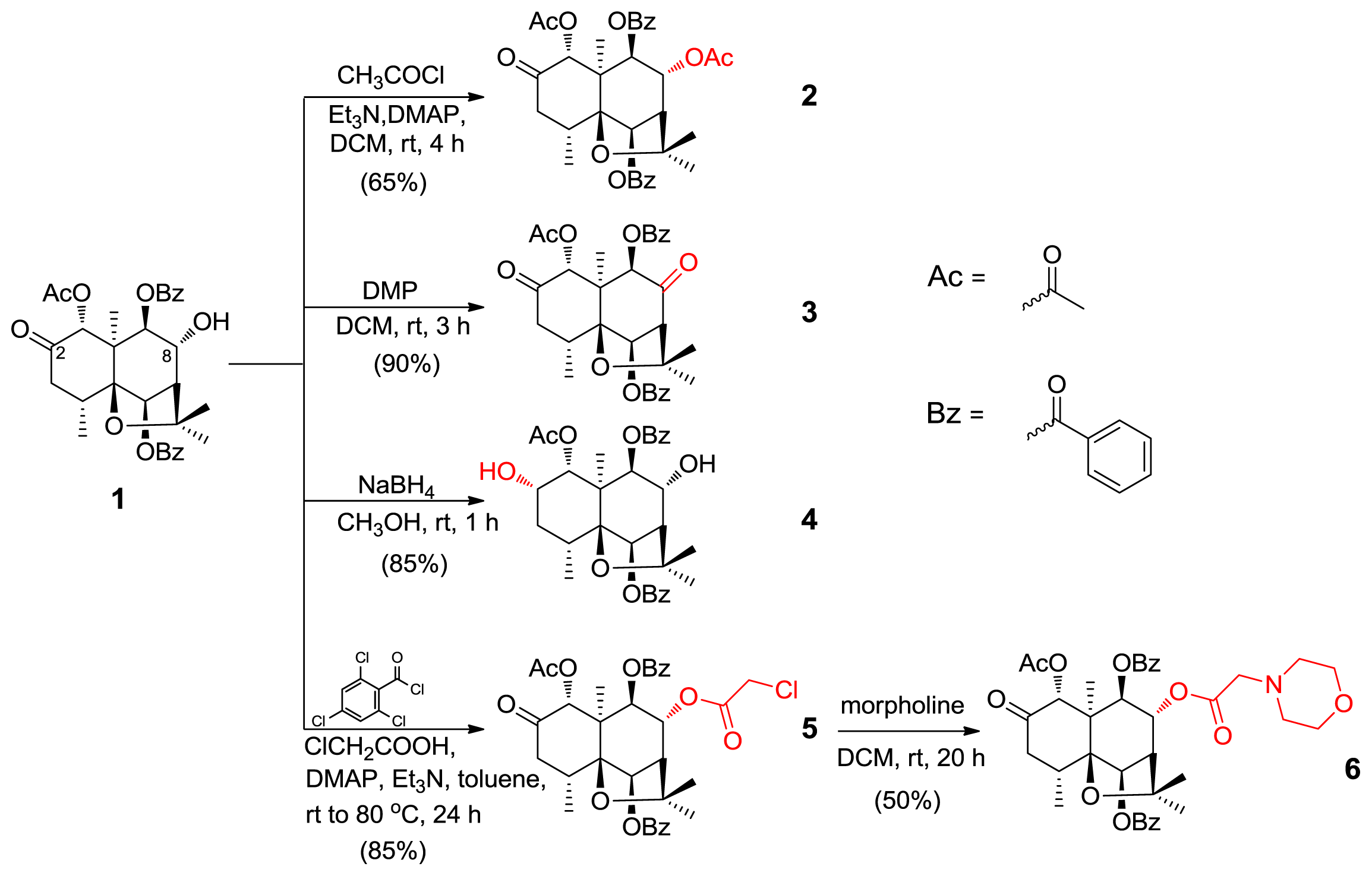
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Semisynthesis of Derivatives (2–6)
3.3. Antifeedant Test
4. Conclusions
Supplementary Information
ijms-14-19484-s001.pdf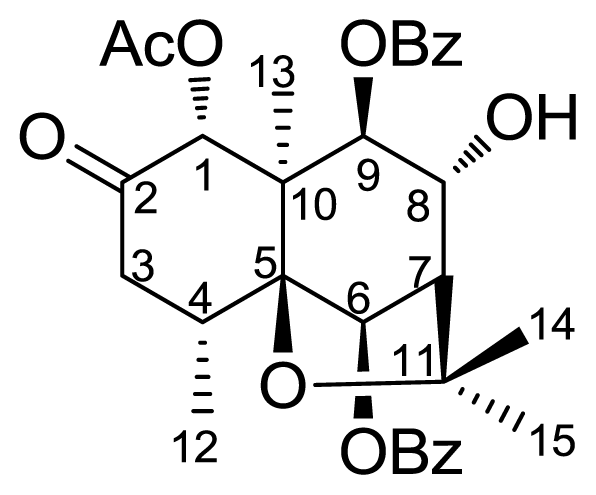
| Position | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| H-1 | 5.95 (s) | 5.96 (s) | 5.56 (d, 3.0) | 5.95 (s) | 5.95 (s) |
| H-2 | 2.09 (d, 3.0) | ||||
| H-3α | 3.39 (dd, 12.5, 7.5) | 3.40 (dd, 13, 7.5) | 3.73 (m) | 3.40 (dd, 12.8, 7.5) | 3.40 (dd, 12.5, 7.5) |
| H-3β | 2.30 (dd, 12.5, 1.5) | 2.37 (d, 13) | 2.55 (m) | 2.32 (d, 13) | 2.32 (d, 13) |
| H-4 | 3.02 (m) | 3.09 (m) | 2.67 (m) | 3.05 (m) | 3.03 (m) |
| H-6 | 6.04 (s) | 5.89 (1H,s) | 6.30 (s) | 5.99 (s) | 5.99 (s) |
| H-7 | 2.76 (d, 3.3) | 3.24 (1H,s) | 2.65 (d, 3.5) | 2.84 (d, 4.0) | 2.72 (d, 3.5) |
| H-8 | 5.39 (d, 3.3) | 4.99 (d, 3.3) | 5.17 (d, 3.3) | 5.08 (d, 3.3) | |
| H-9 | 5.09 (s) | 5.49 (s) | 4.47 (s) | 5.52 (s) | 5.43 (s) |
| H-12 | 1.05 (d, 7.5) | 1.05 (d, 7.5) | 1.36 (d, 8.0) | 1.06 (d, 7.0) | 1.05 (d, 6.5) |
| H-13 | 1.45 (s) | 1.46 (3H,s) | 1.45 (s) | 1.45 (s) | 1.49 (s) |
| H-14 | 1.57 (s) | 1.54 (3H,s) | 1.56 (s) | 1.57 (s) | 1.56 (s) |
| H-15 | 2.29 (s) | 1.62 (3H,s) | 1.62 (s) | 1.61 (s) | 1.62 (s) |
| AcO-8 (CH3) | 1.61 (s) | ||||
| RCH2OCO-8b | 4.35 (s) | 3.46 (s) | |||
| N(CH2CH2)2O | 2.78 (m) | ||||
| N(CH2CH2)2O | 3.57 (m) |
| Position | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| C-1 | 77.4 | 76.7 | 74.9 | 77.5 | 77.4 |
| C-2 | 204.3 | 203.5 | 70.8 | 204.2 | 204.3 |
| C-3 | 43.9 | 44.0 | 34.1 | 43.9 | 43.9 |
| C-4 | 38.7 | 38.2 | 34.0 | 38.7 | 38.7 |
| C-5 | 89.5 | 91.2 | 90.8 | 89.4 | 89.5 |
| C-6 | 76.1 | 70.8 | 76.2 | 76.3 | 76.0 |
| C-7 | 53.1 | 74.0 | 55.7 | 52.9 | 53.2 |
| C-8 | 75.9 | 202.2 | 74.6 | 75.9 | 77.2 |
| C-9 | 77.2 | 65.35 | 80.4 | 76.5 | 76.3 |
| C-10 | 55.0 | 51.9 | 55.6 | 55.1 | 59.4 |
| C-11 | 82.9 | 84.1 | 81.7 | 82.9 | 82.9 |
| C-12 | 18.1 | 17.8 | 18.7 | 18.1 | 14.2 |
| C-13 | 20.2 | 19.9 | 19.4 | 20.3 | 20.3 |
| C-14 | 31.0 | 21.9 | 25.7 | 25.5 | 25.5 |
| C-15 | 25.6 | 27.3 | 29.7 | 31.0 | 31.0 |
| AcO-8 | 21.2, 169.3 | ||||
| RCH2OCO-8 b | 41.0, 172.6 | 55.0, 165.3 | |||
| N(CH2CH2)2O | 53.3 | ||||
| N(CH2CH2)2O | 67.0 |
| Entry | 24 h-Antifeedant rate (%) | 48 h-Antifeedant rate (%) |
|---|---|---|
| control | 0 | 0 |
| 1 | 55 | 45 |
| 2 | 65 | 50 |
| 3 | 15 | 12.5 |
| 4 | 80 | 72.5 |
| 5 | 0 | 0 |
| 6 | 0 | 0 |
| Probenazole | 100 | 97.50 |
Acknowledgments
Conflicts of Interest
References
- Agarwal, M.; Walia, S.; Dhingra, S.; Khambay, B.P.S. Insect growth inhibition, antifeedant and antifungal activity of compounds isolated/derived from Zingiber officinale Roscoe (ginger) rhizomes. Pest. Manag. Sci 2001, 57, 289–300. [Google Scholar]
- Akhtar, Y.; Isman, M.B.; Niehaus, L.A.; Lee, C.-H.; Lee, H.-S. Antifeedant and toxic effects of naturally occurring and synthetic quinones to the cabbage looper Trichoplusia ni. Crop Prot 2012, 31, 8–14. [Google Scholar]
- Gao, J.M.; Wu, W.J.; Zhang, J.W.; Konishi, Y. The dihydro-β-agarofuran sesquiterpenoids. Nat. Prod. Rep 2007, 24, 1153–1189. [Google Scholar]
- Liu, G.Q.; Gao, J.M.; Wu, W.J. Adance in macrocyclic dihydroagarofuran pyriding alkaloids. Chem. Res. Appl 2003, 15, 321–326. [Google Scholar]
- Spivey, A.C.; Weston, M.; Woodhead, S. Celastraceae sesquiterpenoids: Biological activity and synthesis. Chem. Soc. Rev 2002, 31, 43–59. [Google Scholar]
- Kuo, Y.-H.; King, M.-L.; Chen, C.-F.; Chen, H.-Y.; Chen, C.-H.; Chen, K.; Lee, K.-H. Two new macrolide sesquiterpene pyridine alkaloids from Maytenus emarginata: Emarginatine G and the cytotoxic emarginatine F. J. Nat. Prod 1994, 57, 263–269. [Google Scholar]
- Pérez-Victoria, J.M.; Tincusi, B.M.; Jiménez, I.A.; Bazzocchi, I.L.; Gupta, M.P.; Castanys, S.; Gamarro, F.; Ravelo, A.G. New natural sesquiterpenes as modulators of daunomycin resistance in a multidrug-resistant Leishmania tropica Line. J. Med. Chem 1999, 42, 4388–4393. [Google Scholar]
- Duan, H.; Takaishi, Y.; Bando, M.; Kido, M.; Imakura, Y.; Lee, K. Novel sesquiterpene esters with alkaloid and monoterpene and related compounds from Tripterygium hypoglaucum: A new class of potent anti-HIV agents. Tetrahedron Lett 1999, 40, 2969–2972. [Google Scholar]
- Munoz-Martinez, F.; Lu, P.; Cortes-Selva, F.; Perez-Victoria, J.M.; Jimenez, I.A.; Ravelo, A.G.; Sharom, F.J.; Gamarro, F.; Castanys, S. Celastraceae sesquiterpenes as a new class of modulators that bind specifically to human P-glycoprotein and reverse cellular multidrug resistance. Cancer Res 2004, 64, 7130–7138. [Google Scholar]
- Cortés-Selva, F.; Jiménez, I.; Munoz-Martinez, F.; Campillo, M.; Bazzocchi, I.; Pardo, L.; Ravelo, A.; Castanys, S.; Gamarro, F. Dihydro-β-agarofuran sesquiterpenes: A new class of reversal agents of the multidrug resistance phenotype mediated by P-glycoprotein in the protozoan parasite Leishmania. Curr. Pharm. Des 2005, 11, 3125–3139. [Google Scholar]
- Cortés-Selva, F.; Munoz-Martinez, F.; Ilias, A.; Jimenez, A.I.; Varadi, A.; Gamarro, F.; Castanys, S. Functional expression of a multidrug P-glycoprotein transporter of Leishmania. Biochem. Biophys. Res. Commun 2005, 329, 502–507. [Google Scholar]
- Ujita, K.; Takaishi, Y.; Tokuda, H.; Nishino, H.; Iwashima, A.; Fujjita, A.T. Inhibitory effects of triptogelin A-1 on 12-O-tetradecanoylphorbol-13-acetate-induced skin tumor promotion. Cancer Lett 1993, 68, 129–133. [Google Scholar]
- Takaishi, Y.; Ujita, K.; Tokuda, H.; Nishino, H.; Iwashima, A.; Fujita, T. Inhibitory effects of dihydroagarofuran sesquiterpenes on Epstein-Barr virus activation. Cancer Lett 1992, 65, 19–26. [Google Scholar]
- Zhang, J.; Hu, Z.; Li, S.; Wu, W. Synthesis and insecticidal activities of new ester-derivatives of Celangulin-V. Int. J. Mol. Sci 2011, 12, 9596–9604. [Google Scholar]
- Zhang, J.; Hu, Z.; Yang, H.; Wu, W. Synthesis and insecticidal activities of new ether-derivatives of Celangulin-V. Nat. Prod. Commun 2010, 5, 845–848. [Google Scholar]
- Zhang, J.; Li, S.; Ji, Z.; Hu, Z.; Wu, W. Synthesis and insecticidal activity of novel dimers of celangulin-V and podophyllotoxin. Chem. Nat. Compd 2009, 45, 507–510. [Google Scholar]
- Johnson, L.A.; Soltis, D.E. mat K DNA sequences and phylogenetic reconstruction in Saxifragaceaes. str. Syst. Bot 1994, 19, 143–156. [Google Scholar]
- Wang, D.-M.; Li, D.-W.; Pu, W.-J.; Liu, J.-J. Preparation and application of dihydro-β-agarofuran sesquiterpenes from Parnassia wightiana. Wall. CN Patent 103073529, 2013. [Google Scholar]
- Torres-Romero, D.; Jiménez, I.; Rojas, R.; Gilman, R.; López, M.; Bazzocchi, I. Dihydro-β-agarofuran sesquiterpenes isolated from Celastrus vulcanicola as potential anti-Mycobacterium tuberculosis multidrug-resistant agents. Bioorgan. Med. Chem 2011, 19, 2182–2189. [Google Scholar]
- Polak, A. Preclinical data and mode of action of amorolfine. Clin. Exp. Dermatol 1992, 17, 8–12. [Google Scholar]
- Dhimitruka, I.; SantaLucia, J., Jr. Investigation of the Yamaguchi esterification mechanism. Synthesis of a Lux-S enzyme inhibitor using an improved esterification method. Org. Lett 2006, 8, 47–50. [Google Scholar]
- Akhtar, Y.; Isman, M.B.; Paduraru, P.M.; Nagabandi, S.; Nair, R.; Plettner, E. Screening of dialkoxybenzenes and disubstituted cyclopentene derivatives against the cabbage looper, Trichoplusia ni, for the discovery of new feeding and oviposition deterrents. J. Agric. Food Chem 2007, 55, 10323–10330. [Google Scholar]
- Iwai, T.; Seo, S.; Mitsuhara, I.; Ohashi, Y. Probenazole-induced accumulation of salicylic acid confers resistance to Magnaporthe grisea in adult rice plants. Plant Cell Physiol 2007, 48, 915–924. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tang, J.-J.; Zhang, F.-Y.; Wang, D.-M.; Tian, J.-M.; Dong, S.; Gao, J.-M. Semisynthesis and Antifeedant Activity of New Derivatives of a Dihydro-β-Agarofuran from Parnassia wightiana. Int. J. Mol. Sci. 2013, 14, 19484-19493. https://doi.org/10.3390/ijms141019484
Tang J-J, Zhang F-Y, Wang D-M, Tian J-M, Dong S, Gao J-M. Semisynthesis and Antifeedant Activity of New Derivatives of a Dihydro-β-Agarofuran from Parnassia wightiana. International Journal of Molecular Sciences. 2013; 14(10):19484-19493. https://doi.org/10.3390/ijms141019484
Chicago/Turabian StyleTang, Jiang-Jiang, Fei-Yu Zhang, Dong-Mei Wang, Jun-Mian Tian, Shuai Dong, and Jin-Ming Gao. 2013. "Semisynthesis and Antifeedant Activity of New Derivatives of a Dihydro-β-Agarofuran from Parnassia wightiana" International Journal of Molecular Sciences 14, no. 10: 19484-19493. https://doi.org/10.3390/ijms141019484




