Kaempferol Promotes Apoptosis in Human Bladder Cancer Cells by Inducing the Tumor Suppressor, PTEN
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
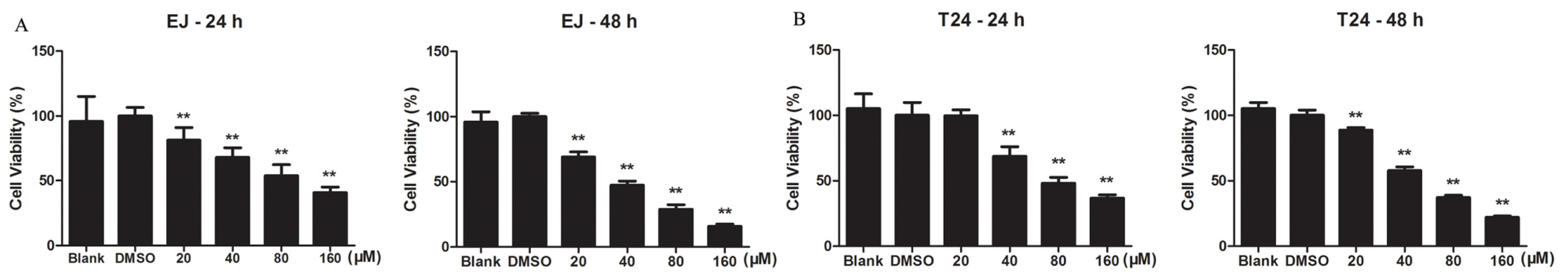
2.1.1. Kae Inhibits the Viability of Bladder Cancer Cells
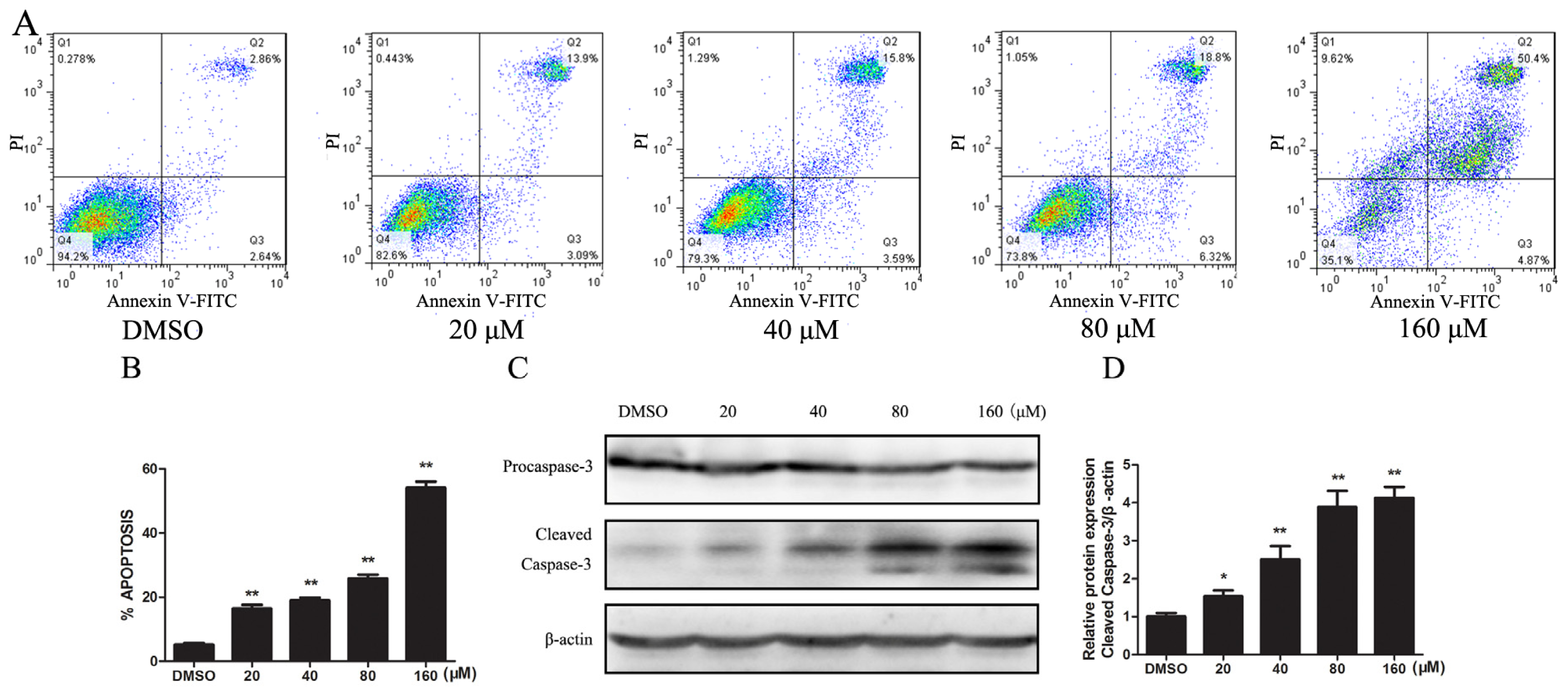
2.1.2. Kae Induces Apoptosis by Activating the Caspase-3 Pathway in EJ Cells
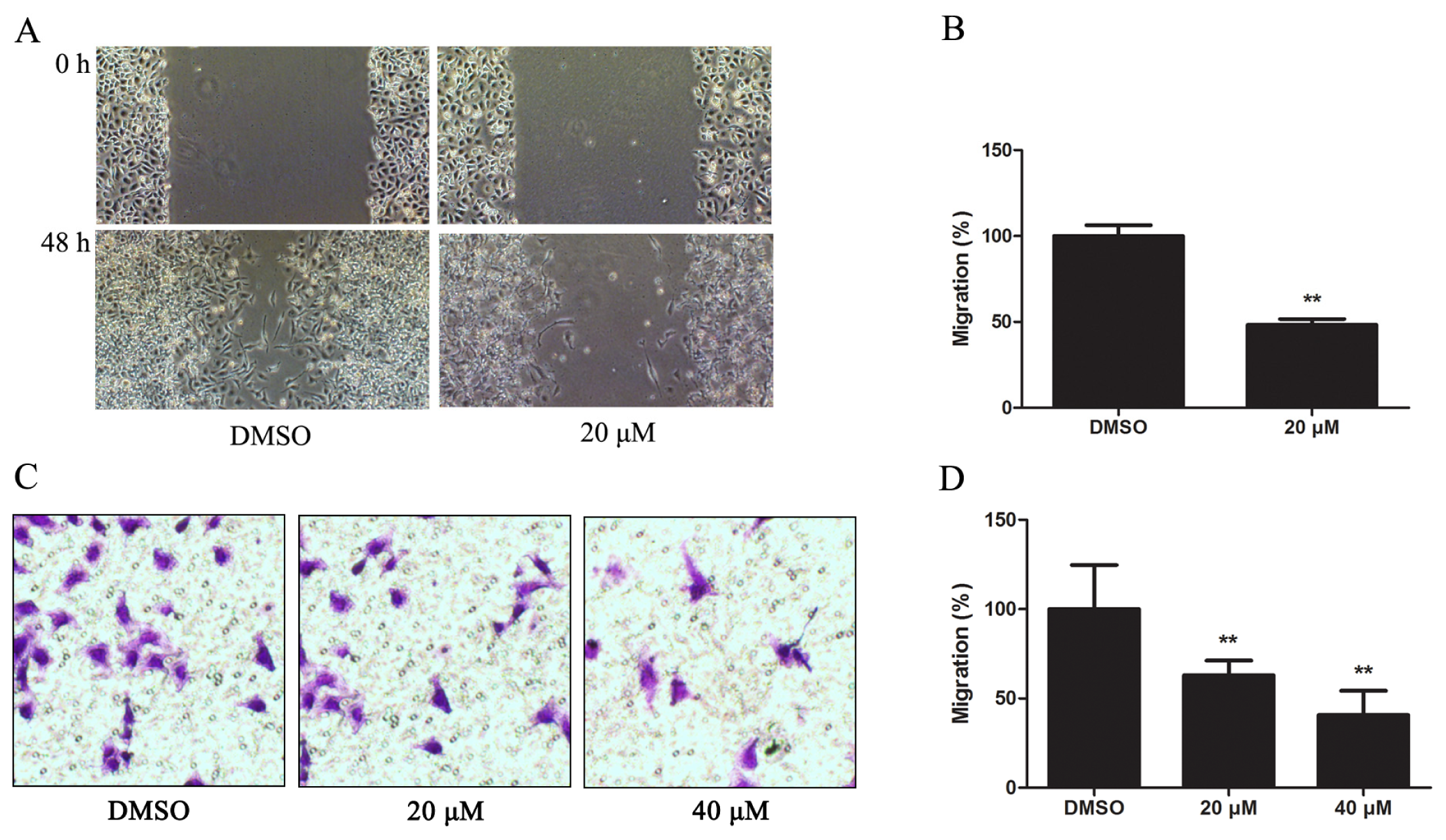
2.1.3. Kae Suppresses Migration of EJ Cells
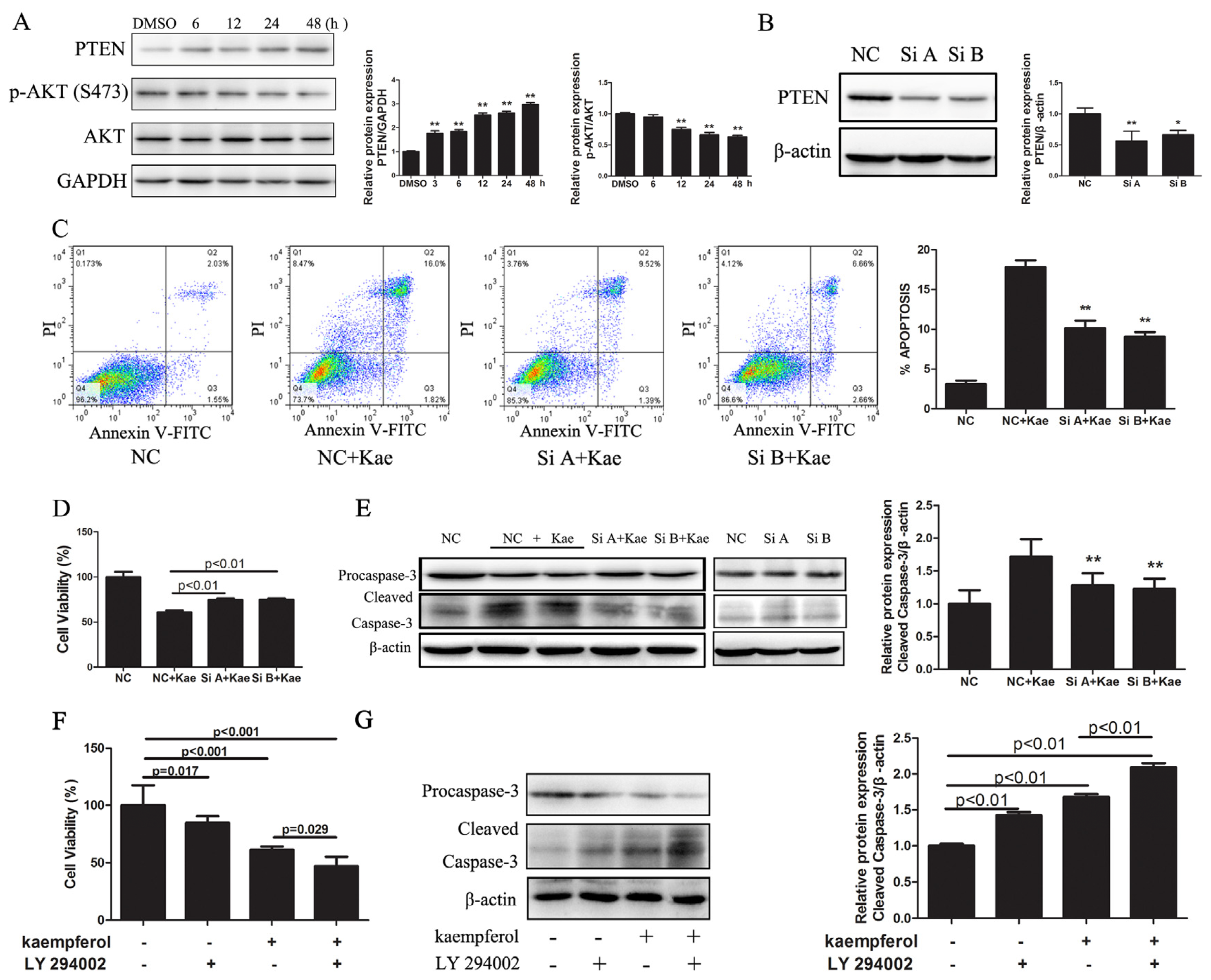
2.1.4. Kae-Induced Apoptosis in EJ Cells Involves PTEN
2.2. Discussion
3. Experimental Section
3.1. Reagents and Cell Culture
3.2. Cell Viability Assay
3.3. Apoptosis Analysis
3.4. Western Blot Analysis
3.5. Wound-Healing Assay
3.6. Transwell Cell Migration Assay
3.7. Small Interfering RNA-Mediated Knockdown of PTEN
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Falke, J.; Witjes, J.A. Contemporary management of low-risk bladder cancer. Nat. Rev. Urol 2011, 8, 42–49. [Google Scholar]
- Gonzalez, C.A.; Riboli, E. Diet and cancer prevention: Where we are, where we are going. Nutr. Cancer 2006, 56, 225–231. [Google Scholar]
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A 1998, 799, 101–110. [Google Scholar]
- Kuhlmann, F.; Müller, C. Independent responses to ultraviolet radiation and herbivore attack in broccoli. J. Exp. Bot 2009, 60, 3467–3475. [Google Scholar]
- Sampson, L.; Rimm, E.; Hollman, P.C.; de Vries, J.H.; Katan, M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc 2002, 102, 1414–1420. [Google Scholar]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol 2007, 224, 274–283. [Google Scholar]
- Kris-Etherton, P.M.; Keen, C.L. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr. Opin. Lipidol 2002, 13, 41–49. [Google Scholar]
- Hertog, M.G.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr. Cancer 1993, 20, 21–29. [Google Scholar]
- Leung, H.W.; Lin, C.J.; Hour, M.J.; Yang, W.H.; Wang, M.Y.; Lee, H.Z. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem. Toxicol 2007, 45, 2005–2013. [Google Scholar]
- Luo, H.; Rankin, G.O.; Li, Z.; Depriest, L.; Chen, Y.C. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem 2011, 128, 513–519. [Google Scholar]
- Marfe, G.; Tafani, M.; Indelicato, M.; Sinibaldi-Salimei, P.; Reali, V.; Pucci, B.; Fini, M.; Russo, M.A. Kaempferol induces apoptosis in two different cell lines via Akt inactivation, Bax and SIRT3 activation, and mitochondrial dysfunction. J. Cell. Biochem 2009, 106, 643–650. [Google Scholar]
- De Leo, M.; Braca, A.; Sanogo, R.; Cardile, V.; DeTommasi, N.; Russo, A. Antiproliferative activity of Pteleopsis suberosa leaf extract and its flavonoid components in human prostate carcinoma cells. Planta Med 2006, 72, 604–610. [Google Scholar]
- Kang, G.Y.; Lee, E.R.; Kim, J.H.; Jung, J.W.; Lim, J.; Kim, S.K.; Cho, S.G.; Kim, K.P. Downregulation of PLK-1 expression in kaempferol-induced apoptosis of MCF-7 cells. Eur. J. Pharmacol 2009, 611, 17–21. [Google Scholar]
- Mutoh, M.; Takahashi, M.; Fukuda, K.; Matsushima-Hibiya, Y.; Mutoh, H.; Sugimura, T.; Wakabayashi, K. Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis 2000, 21, 959–963. [Google Scholar]
- Aveyard, J.S.; Skilleter, A.; Habuchi, T.; Knowles, M.A. Somatic mutation of PTEN in bladder carcinoma. Br. J. Cancer 1999, 80, 904–908. [Google Scholar]
- Jiang, Z.; Pore, N.; Cerniglia, G.J.; Mick, R.; Georgescu, M.M.; Bernhard, E.J.; Hahn, S.M.; Gupta, A.K.; Maity, A. Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer Res 2007, 67, 4467–4473. [Google Scholar]
- Lee, S.; Choi, E.J.; Jin, C.; Kim, D.H. Activation of PI3 K/Akt pathway by PTEN reduction and PIK3CA mRNA ampliWcation contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol. Oncol 2005, 97, 26–34. [Google Scholar]
- Dahia, P.L. PTEN, a unique tumor suppressor gene. Endocr. Relat. Cancer 2000, 7, 115–129. [Google Scholar]
- Aligiannis, N.; Mitaku, S.; Mitrocotsa, D.; Leclerc, S. Flavonoids as cycline-dependent kinase inhibitors: Inhibition of cdc 25 phosphatase activity by flavonoids belonging to the quercetin and kaempferol series. Planta Med 2001, 67, 468–470. [Google Scholar]
- Dimas, K.; Demetzos, C.; Mitaku, S.; Marselos, M.; Tzavaras, T.; Kokkinopoulos, D. Cytotoxic activity of kaempferol glycosides against human leukaemic cell linesin vitro. Pharmacol. Res. 2000, 41, 85–88. [Google Scholar]
- Kelly, P.N.; Strasser, A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ 2011, 18, 1414–1424. [Google Scholar]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar]
- Kaufmann, S.H.; Vaux, D.L. Alterations in the apoptotic machinery and their potential role in anticancer drug resistance. Oncogene 2003, 22, 7414–7430. [Google Scholar]
- Reed, J.C. Apoptosis-based therapies. Nat. Rev. Drug Discov 2002, 1, 111–121. [Google Scholar]
- Earnshaw, W.C.; Martins, L.M.; Kaufmann, S.H. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem 1999, 68, 383–424. [Google Scholar]
- Liotta, L.A.; Stetler-Stevenson, W.G. Tumor invasion and metastasis: An imbalance of positive and negative regulation. Cancer Res 1991, 51, 5054s–5059s. [Google Scholar]
- Phromnoi, K.; Yodkeeree, S.; Anuchapreeda, S.; Limtrakul, P. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol. Sin 2009, 30, 1169–1176. [Google Scholar]
- Zhang, X.M.; Huang, S.P.; Xu, Q. Quercetin inhibits the invasion of murine melanoma B16-BL6 cells by decreasing pro-MMP-9 via the PKC pathway. Cancer Chemother. Pharmacol 2004, 53, 82–88. [Google Scholar]
- Steck, P.A.; Pershouse, M.A.; Jasser, S.A.; Yung, W.K.; Lin, H.; Ligon, A.H.; Langford, L.A.; Baumgard, M.L.; Hattier, T.; Davis, T.; et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet 1997, 15, 356–362. [Google Scholar]
- Puzio-Kuter, A.M.; Castillo-Martin, M.; Kinkade, C.W.; Wang, X.; Shen, T.H.; Matos, T.; Shen, M.M.; Cordon-Cardo, C.; Abate-Shen, C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev 2009, 23, 675–680. [Google Scholar]
- Di Cristofano, A.; Pandolfi, P.P. The multiple roles of PTEN in tumor suppression. Cell 2000, 100, 387–390. [Google Scholar]
- Wu, X.; Obata, T.; Khan, Q.; Highshaw, R.A.; de Vere White, R.; Sweeney, C. The phosphatidylinositol-3 kinase pathway regulates bladder cancer cell invasion. BJU Int 2004, 93, 143–150. [Google Scholar]
- Tanaka, M.; Koul, D.; Davies, M.A.; Liebert, M.; Steck, P.A.; Grossman, H.B. MMAC1/PTEN inhibits cell growth and induces chemosensitivity to doxorubicin in human bladder cancer cells. Oncogene 2000, 19, 5406–5412. [Google Scholar]
- Garcia, R.; Gonzalez, C.A.; Agudo, A.; Riboli, E. High intake of specific carotenoids and flavonoids does not reduce the risk of bladder cancer. Nutr. Cancer 1999, 35, 212–214. [Google Scholar]
- Rajbhandari, R.; Peng, N.; Moore, R.; Arabshahi, A.; Wyss, J.M.; Barnes, S.; Prasain, J.K. Determination of cranberry phenolic metabolites in rats by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem 2011, 59, 6682–6688. [Google Scholar]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.; Kong, A.N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos 2009, 30, 356–365. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xie, F.; Su, M.; Qiu, W.; Zhang, M.; Guo, Z.; Su, B.; Liu, J.; Li, X.; Zhou, L. Kaempferol Promotes Apoptosis in Human Bladder Cancer Cells by Inducing the Tumor Suppressor, PTEN. Int. J. Mol. Sci. 2013, 14, 21215-21226. https://doi.org/10.3390/ijms141121215
Xie F, Su M, Qiu W, Zhang M, Guo Z, Su B, Liu J, Li X, Zhou L. Kaempferol Promotes Apoptosis in Human Bladder Cancer Cells by Inducing the Tumor Suppressor, PTEN. International Journal of Molecular Sciences. 2013; 14(11):21215-21226. https://doi.org/10.3390/ijms141121215
Chicago/Turabian StyleXie, Feng, Ming Su, Wei Qiu, Min Zhang, Zhongqiang Guo, Boxing Su, Jie Liu, Xuesong Li, and Liqun Zhou. 2013. "Kaempferol Promotes Apoptosis in Human Bladder Cancer Cells by Inducing the Tumor Suppressor, PTEN" International Journal of Molecular Sciences 14, no. 11: 21215-21226. https://doi.org/10.3390/ijms141121215



