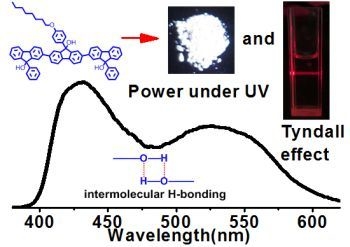
Supramolecular Luminescence from Oligofluorenol-Based Supramolecular Polymer Semiconductors
Abstract
:1. Introduction
2. Results and Discussion
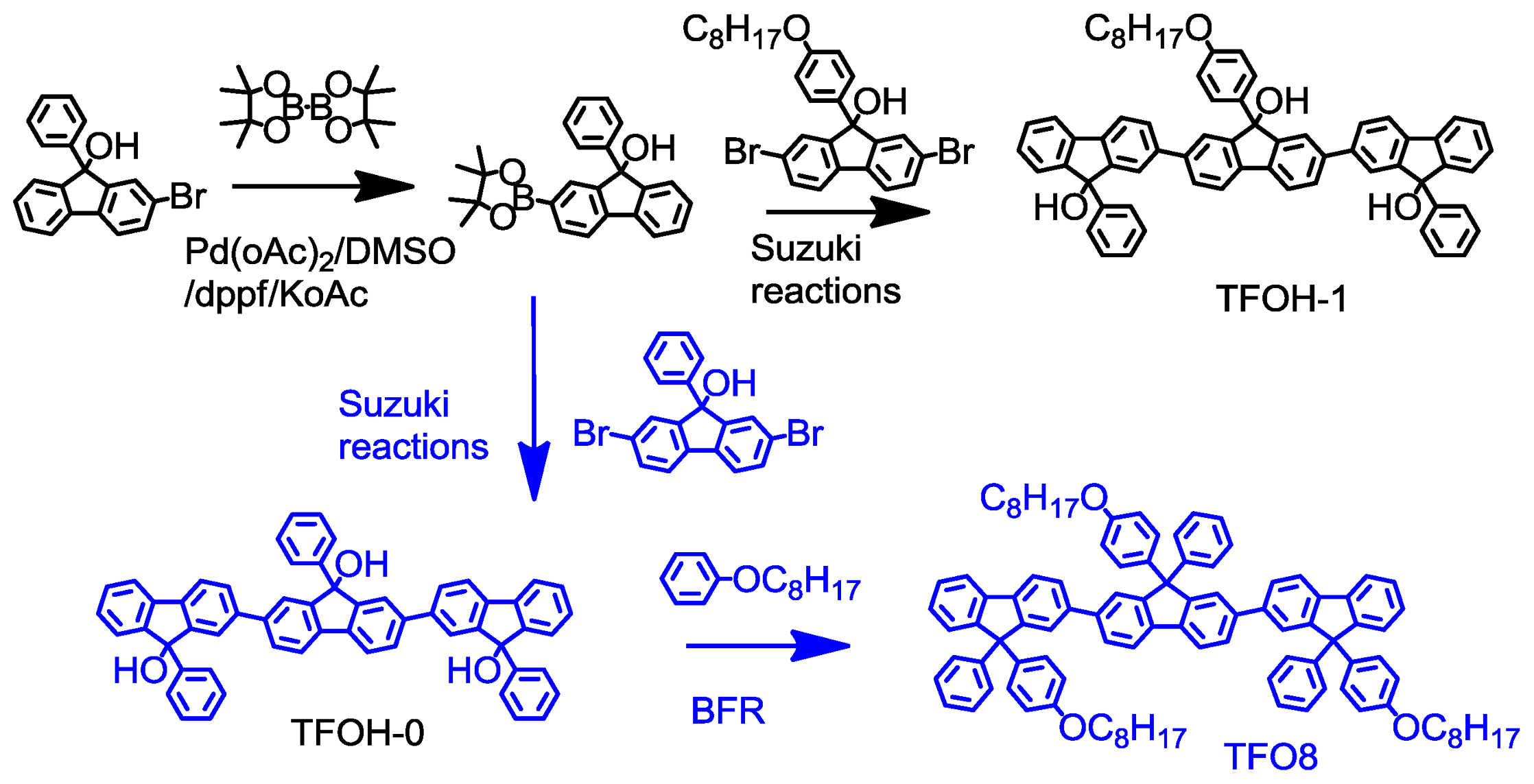
2.1. Synthesis
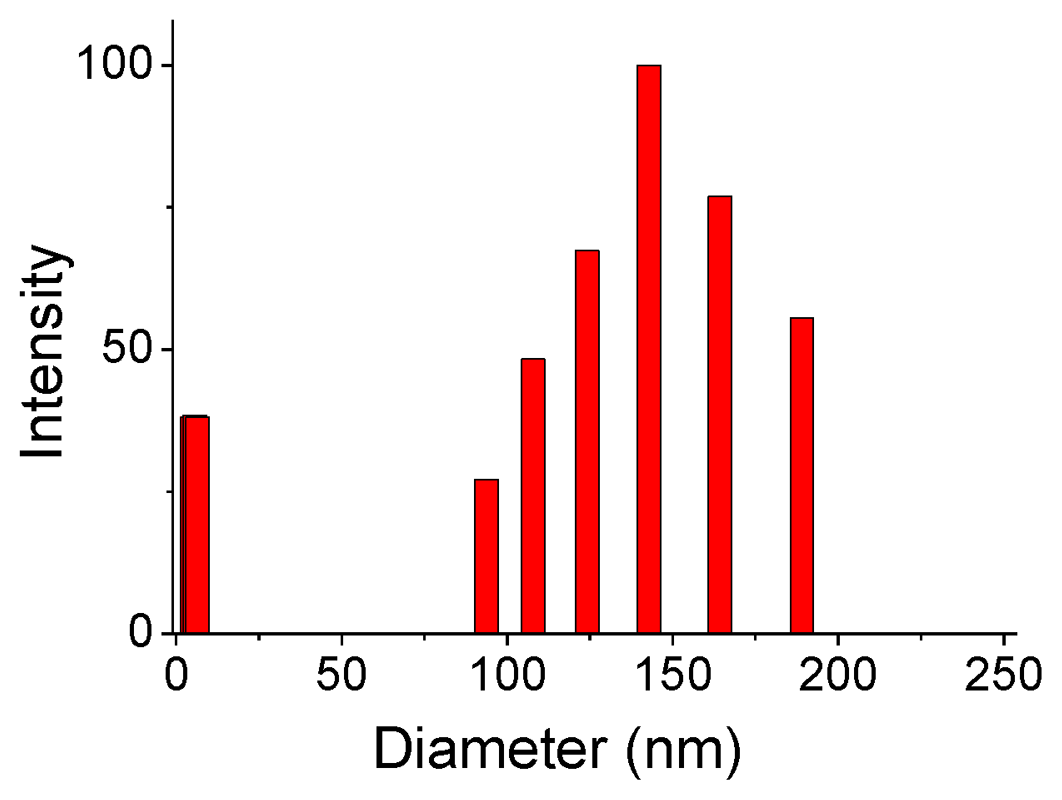
2.2. Supramolecular Polymers and Aggregates in Solutions
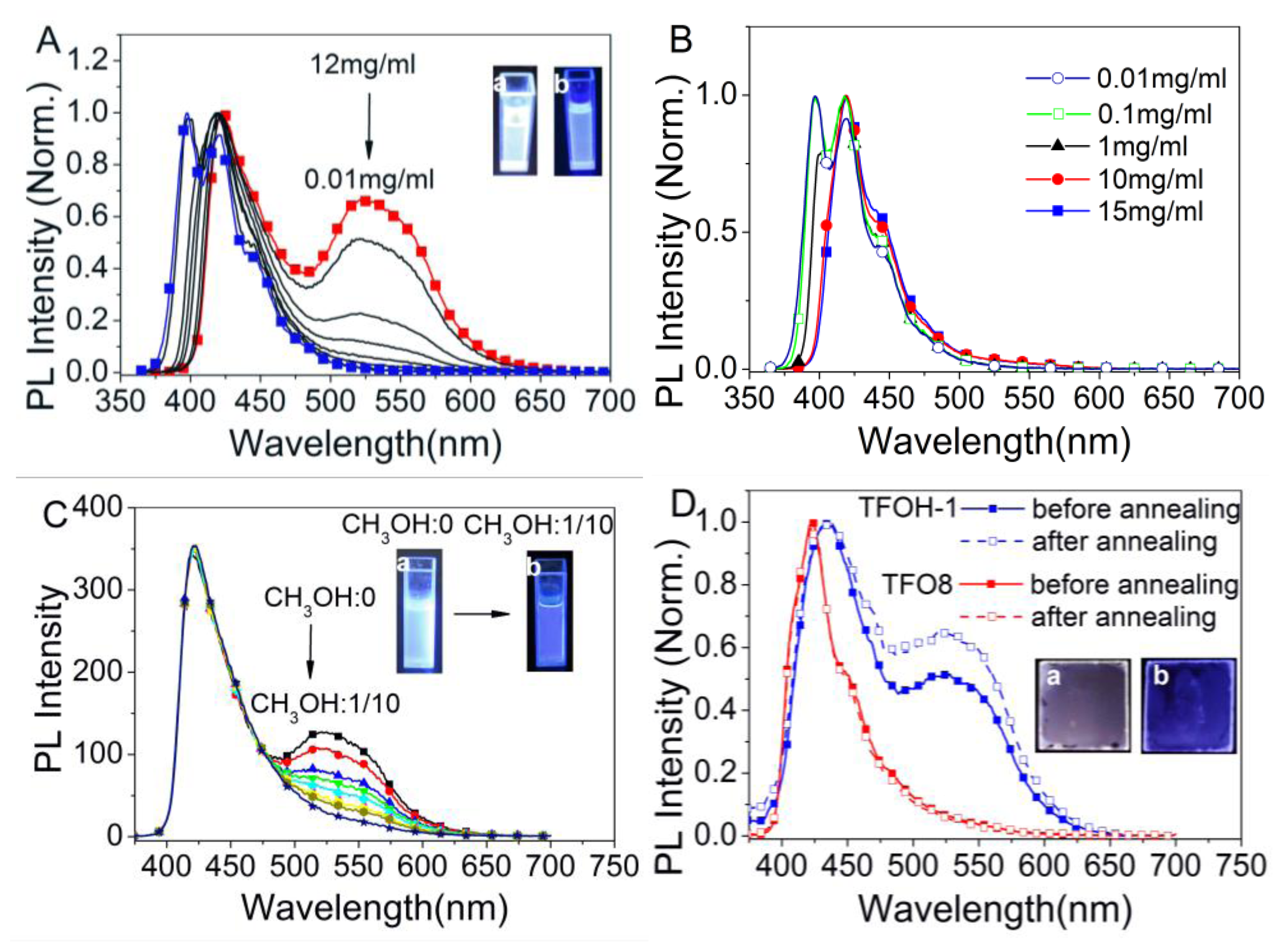
2.3. Supramolecular Luminescence in Solution and Thin Films
3. Experimental Section
3.1. Chemicals
3.2. Characterization
3.3. Synthesis
3.3.1. Synthesis of 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9-phenylfluoren-9-ol (TMB-PFOH) from 2-bromo-9-phenylfluoren-9-ol (BrPFOH)
3.3.2. Synthesis of TFOH-1 via Suzuki coupling reaction
3.3.3. Synthesis of 9,9′,9″-triphenyl-[2,2′:7′,2″-terfluorene]-9,9′,9″-triol (TFOH-0)
3.3.4. Synthesis of 9,9′,9″-tris(4-(octyloxy)phenyl)-9,9′,9″-triphenyl-2,2′:7′,2″-terfluorene (TFO8)
4. Conclusions
Supplementary Information
ijms-14-22368-s001.pdf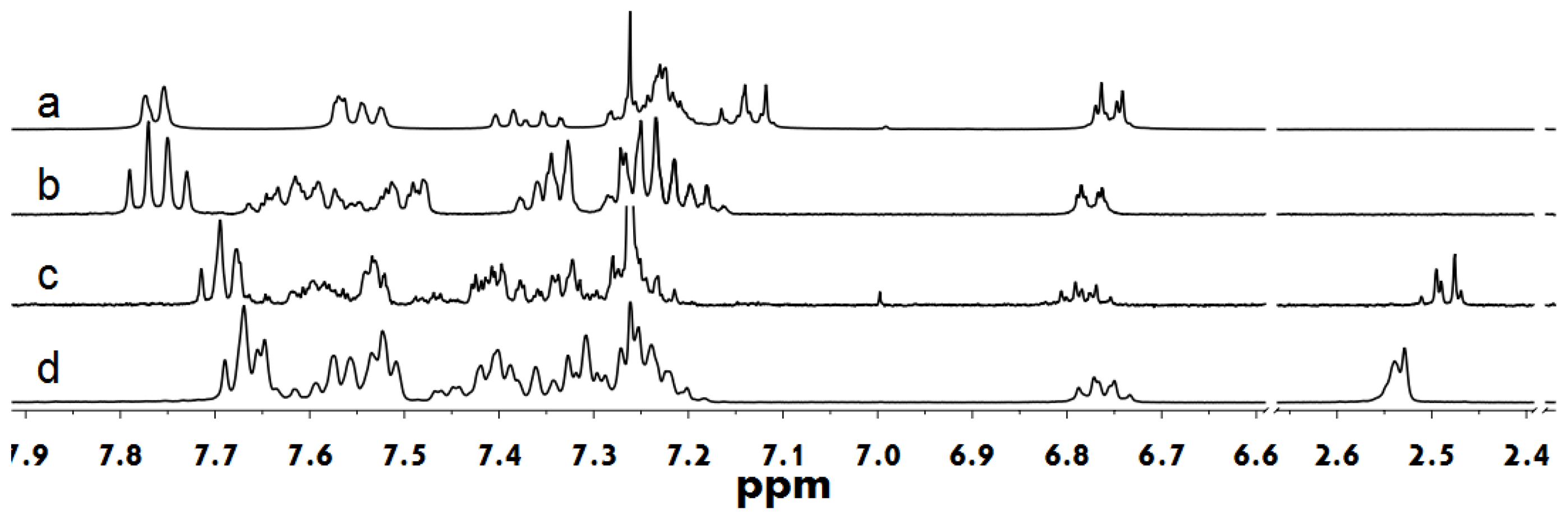

Acknowledgments
Conflicts of Interest
References
- Xie, L.H.; Yin, C.R.; Lai, W.Y.; Fan, Q.L.; Huang, W. Polyfluorene-based semiconductors combined with various periodic table elements for organic electronics. Progr. Polym. Sci 2012, 37, 1192–1264. [Google Scholar]
- Xie, L.-H.; Chang, Y.-Z.; Gu, J.-F.; Sun, R.-J.; Li, J.-W.; Zhao, X.-H.; Huang, W. Design of organic/polymeric π-Semiconductors: The four-element principle. Acta Phys. Chim. Sin 2010, 26, 1784–1794. [Google Scholar]
- Abbel, R.; Schenning, A.P.H.J.; Meijer, E.W. Fluorene-based materials and their supramolecular properties. J. Polym. Sci. Part A 2009, 47, 4215–4233. [Google Scholar]
- Yin, C.R.; Ye, S.H.; Zhao, J.; Yi, M.D.; Xie, L.H.; Lin, Z.Q.; Chang, Y.Z.; Liu, F.; Xu, H.; Shi, N.E.; et al. Hindrance-functionalized pi-Stacked polymer host materials of the cardo-type carbazole-fluorene hybrid for solution-processable blue electrophosphorescent devices. Macromolecules 2011, 44, 4589–4595. [Google Scholar]
- Yin, C.-R.; Han, Y.; Li, L.; Ye, S.-H.; Mao, W.-W.; Yi, M.-D.; Ling, H.-F.; Xie, L.-H.; Zhang, G.-W.; Huang, W. Hindrance-functionalized [small pi]-stacked polymer based on polystyrene with pendent cardo groups for organic electronics. Polym. Chem 2013, 4, 2540–2545. [Google Scholar]
- Xie, L.H.; Deng, X.Y.; Chen, L.; Chen, S.F.; Liu, R.R.; Hou, X.Y.; Wong, K.Y.; Ling, Q.D.; Huang, W. A pi-Stacked and conjugated hybrid based on Poly(N-vinylcarbazole) postfunctionalized with terfluorene for stable deep-blue hole-transporting materials. J. Polym. Sci. Pol. Chem 2009, 47, 5221–5229. [Google Scholar]
- Li, W.-J.; Liu, B.; Qian, Y.; Xie, L.-H.; Wang, J.; Li, S.-B.; Huang, W. Synthesis and characterization of diazafluorene-based oligofluorenes and polyfluorene. Polym. Chem 2013, 4, 1796–1802. [Google Scholar]
- Molla, M.R.; Ghosh, S. Hydrogen-bonding-mediated J-aggregation and white-light emission from a remarkably simple, single-component, naphthalenediimide chromophore. Chem. Eur. J 2012, 18, 1290–1294. [Google Scholar]
- Abbel, R.; Grenier, C.; Pouderoijen, M.J.; Stouwdam, J.W.; Leclère, P.E.L.G.; Sijbesma, R.P.; Meijer, E.W.; Schenning, A.P.H.J. White-light emitting hydrogen-bonded supramolecular copolymers based on π-conjugated oligomers. J. Am. Chem. Soc 2008, 131, 833–843. [Google Scholar]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev 2001, 101, 4071–4098. [Google Scholar]
- El-ghayoury, A.; Schenning, A.P.H.J.; van Hal, P.A.; van Duren, J.K.J.; Janssen, R.A.J.; Meijer, E.W. Supramolecular hydrogen-bonded oligo(p-phenylene vinylene) polymers. Angew. Chem. Int. Ed 2001, 40, 3660–3663. [Google Scholar]
- Ten Cate, A.T.; Kooijman, H.; Spek, A.L.; Sijbesma, R.P.; Meijer, E.W. Conformational control in the cyclization of hydrogen-bonded supramolecular polymers. J. Am. Chem. Soc 2004, 126, 3801–3808. [Google Scholar]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional supramolecular polymers. Science 2012, 335, 813–817. [Google Scholar]
- Zhao, J.F.; Li, Y.B.; Lin, Z.Q.; Xie, L.H.; Shi, N.E.; Wu, X.K.; Wang, C.; Huang, W. Molecule length directed self-assembly behavior of tetratopic oligomeric phenylene-ethynylenes end-capped with carboxylic groups by scanning tunneling microscopy. J. Phys. Chem. C 2010, 114, 9931–9937. [Google Scholar]
- Lin, J.; Yu, Z.; Zhu, W.; Xing, G.; Lin, Z.; Yang, S.; Xie, L.; Niu, C.; Huang, W. A [small π]-conjugated polymer gelator from polyfluorene-based poly(tertiary alcohol) via the hydrogen-bonded supramolecular functionalization. Polym. Chem 2013, 4, 477–483. [Google Scholar]
- Liang, J.; Qian, Y.; Xie, L.-H.; Shi, N.-E.; Chen, S.-F.; Deng, X.-Y.; Huang, W. Spectral stability of polyfluorene-based semiconductors. Acta Phys. Chim. Sin 2010, 26, 946–963. [Google Scholar]
- Xie, L.H.; Hou, X.Y.; Hua, Y.R.; Huang, Y.Q.; Zhao, B.M.; Liu, F.; Peng, B.; Wei, W.; Huang, W. An effective strategy to tune supramolecular interaction via a spiro-bridged spacer in oligothiophene-S,S-dioxides and their anomalous photoluminescent behavior. Org. Lett 2007, 9, 1619–1622. [Google Scholar]
- Wallace, J.U.; Chen, S.H. Fluorene-Based Conjugated Oligomers for Organic Photonics and Electronics. In Polyfluorenes; Scherf, U., Neher, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 212, pp. 145–186. [Google Scholar]
- Egbert Zojer, A.P.; Hennebicq, E.; Beljonne, D.; Brédas, J.-L.; de Freitas, P.S.; Scherf, U.; List, E.J.W. Green emission from poly(fluorene)s: The role of oxidation. J. Chem. Phys 2002, 117, 6794. [Google Scholar]
- Xie, L.-H.; Hou, X.-Y.; Hua, Y.-R.; Tang, C.; Liu, F.; Fan, Q.-L.; Huang, W. Facile synthesis of complicated 9,9-diarylfluorenes based on BF3 center dot Et2O-mediated Friedel-Crafts reaction. Org. Lett 2006, 8, 3701–3704. [Google Scholar]
- Ajayaghosh, A.; George, S.J. First phenylenevinylene based organogels: Self-assembled nanostructures via cooperative hydrogen bonding and π-stacking. J. Am. Chem. Soc 2001, 123, 5148–5149. [Google Scholar]
- Chen, J.; Liu, H.; Weimer, W.A.; Halls, M.D.; Waldeck, D.H.; Walker, G.C. Noncovalent engineering of carbon nanotube surfaces by rigid, functional conjugated polymers. J. Am. Chem. Soc 2002, 124, 9034–9035. [Google Scholar]
- Fernández, G.; Pérez, E.M.; Sánchez, L.; Martín, N. Self-organization of electroactive materials: A head-to-tail donor-acceptor supramolecular polymer. Angew. Chem. Int. Ed 2008, 47, 1094–1097. [Google Scholar]
- Xie, L.H.; Hou, X.Y.; Tang, C.; Hua, Y.R.; Wang, R.J.; Chen, R.F.; Fan, Q.L.; Wang, L.H.; Wei, W.; Peng, B.; et al. Novel H-shaped persistent architecture based on a dispirlo building block system. Org. Lett 2006, 8, 1363–1366. [Google Scholar]
- List, E.J.W.; Guentner, R.; de Freitas, P.S.; Scherf, U. The effect of keto defect sites on the emission properties of polyfluorene-type materials. Adv. Mater 2002, 14, 374–378. [Google Scholar]
- Gong, X.; Iyer, P.K.; Moses, D.; Bazan, G.C.; Heeger, A.J.; Xiao, S.S. Stabilized blue emission from polyfluorene-based light-emitting diodes: Elimination of fluorenone defects. Adv. Funct. Mater 2003, 13, 325–330. [Google Scholar]
- Lemmera, U.; Heun, S.; Mahrta, R.F.; Scherfb, U.; Hopmeiera, M.; Siegnera, U. Aggregate fluorescence in conjugated polymers. Chem. Phys. Lett 1995, 240, 373–378. [Google Scholar]
- Bliznyuk, V.N.; Carter, S.A.; Scott, J.C.; Klärner, G.; Miller, R.D.; Miller, D.C. Electrical and photoinduced degradation of polyfluorene based films and light-emitting devices. Macromolecules 1998, 32, 361–369. [Google Scholar]
- Zeng, G.; Yu, W.-L.; Chua, S.-J.; Huang, W. Spectral and thermal spectral stability study for fluorene-based conjugated polymers. Macromolecules 2002, 35, 6907–6914. [Google Scholar]
- Koizumi, Y.; Seki, S.; Tsukuda, S.; Sakamoto, S.; Tagawa, S. Self-condensed nanoparticles of oligofluorenes with water-soluble side chains. J. Am. Chem. Soc 2006, 128, 9036–9037. [Google Scholar]
- Pei, J.; Liu, X.-L.; Chen, Z.-K.; Zhang, X.-H.; Lai, Y.-H.; Huang, W. First hydrogen-bonding-induced self-assembled aggregates of a polyfluorene derivative. Macromolecules 2002, 36, 323–327. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, G.-W.; Wang, L.; Xie, L.-H.; Lin, J.-Y.; Huang, W. Supramolecular Luminescence from Oligofluorenol-Based Supramolecular Polymer Semiconductors. Int. J. Mol. Sci. 2013, 14, 22368-22379. https://doi.org/10.3390/ijms141122368
Zhang G-W, Wang L, Xie L-H, Lin J-Y, Huang W. Supramolecular Luminescence from Oligofluorenol-Based Supramolecular Polymer Semiconductors. International Journal of Molecular Sciences. 2013; 14(11):22368-22379. https://doi.org/10.3390/ijms141122368
Chicago/Turabian StyleZhang, Guang-Wei, Long Wang, Ling-Hai Xie, Jin-Yi Lin, and Wei Huang. 2013. "Supramolecular Luminescence from Oligofluorenol-Based Supramolecular Polymer Semiconductors" International Journal of Molecular Sciences 14, no. 11: 22368-22379. https://doi.org/10.3390/ijms141122368