An Exploratory Evaluation of Tyrosine Hydroxylase Inhibition in Planaria as a Model for Parkinsonism
Abstract
:1. Introduction
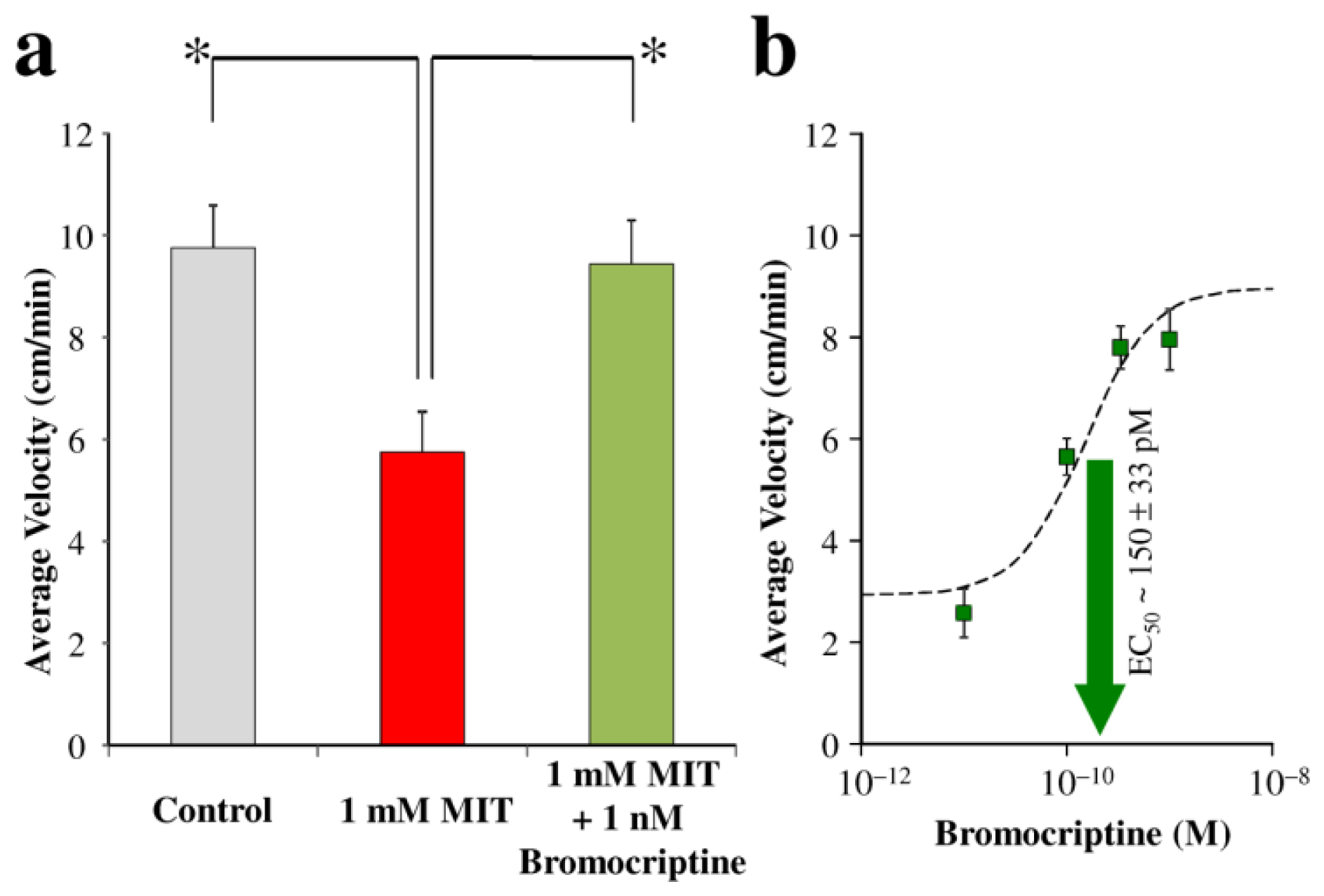
2. Results and Discussion
3. Experimental Section
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Swinney, D.C.; Anthony, J. How were new medicines discovered? Nat. Rev. Drug Discovery 2011, 10, 507–519. [Google Scholar]
- Sams-Dodd, F. Is poor research the cause of the declining productivity of the pharmaceutical industry? An industry in need of a paradigm shift. Drug Discovery Today 2013, 18, 211–217. [Google Scholar]
- Etzion, Y.; Muslin, A.J. The application of phenotypic high-throughput screening techniques to cardiovascular research. Trends Cardiovasc. Res 2009, 19, 207–212. [Google Scholar]
- Umesono, Y.; Agata, K. Evolution and regeneration of the planarian central nervous system. Dev. Growth Differ 2009, 51, 185–195. [Google Scholar]
- Nishimura, K.; Kitamura, Y.; Agata, K. Regeneration of Brain and Dopaminergic Neurons Utilizing Pluripotent Stem Cells: Lessons from Planarians. In Neural Stem Cells and Therapy; Sun, T., Ed.; InTech: Rijeka, Croatia, 2012; pp. 141–158. [Google Scholar]
- Eriksson, K.S.; Panula, P. Gamma-aminobutyric acid in the nervous system of a planarian. J. Comp. Neurol 1994, 345, 528–536. [Google Scholar]
- Carolei, A.; Margotta, V.; Palladini, G. Proposal of a new model with dopaminergic–cholinergic interactions for neuropharmacological investigations. Neuropsychobiology 1975, 1, 355–364. [Google Scholar]
- Venturini, G.; Stocchi, F.; Margotta, V.; Ruggieri, S.; Bravi, D.; Bellantuono, P.; Palladini, G. A pharmacological study of dopaminergic receptor in Planaria. Neuropharmacology 1989, 28, 1377–1382. [Google Scholar]
- Raffa, R.B.; Holland, L.J.; Schulingkamp, R.J. Quantitative assessment of dopamine D2 antagonist activity using invertebrate (Planaria) locomotion as a functional endpoint. Pharmacol. Toxicol. Methods 2001, 45, 223–226. [Google Scholar]
- Talbot, J.; Schötz, E.M. Quantitative characterization of planarian wild-type behavior as a platform for screening locomotion phenotypes. J. Exp. Biol 2010, 214, 1063–1067. [Google Scholar]
- Nagatsu, T.; Sawada, M. Cellular and molecular mechanisms of Parkinson’s disease: Neurotoxins, causative genes, and inflammatory cytokines. Cell. Mol. Neurobiol 2006, 26, 781–802. [Google Scholar]
- Schapira, A.H.V. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol. Sci 2009, 30, 41–47. [Google Scholar]
- Kitamura, Y.; Shimohama, S.; Akaike, A.; Taniguchi, T. The parkinsonian models: Invertebrates to mammals. Jpn. J. Pharmacol 2000, 84, 237–243. [Google Scholar]
- Raffa, R.B.; Danah, J.; Tallarida, C.S.; Zimmerman, C.; Gill, G.; Baron, S.J.; Rawls, S.M. Potential of a planarian model to study certain aspects of anti-Parkinsonism drugs. Adv. Parkinson’s Res 2013, 2, 70–74. [Google Scholar]
- Kitamura, Y.; Kakimura, J.; Taniguchi, T. Protective effect of talipexole on MTPT-treated planarian, a unique parkinsonian worm model. Jpn. J. Pharmacol 1998, 78, 23–29. [Google Scholar]
- Nishimura, K.; Inoue, T.; Yoshimoto, K.; Taniguchi, T.; Kitamura, Y.; Agata, K. Regeneration of dopaminergic neurons after 6-hydroxydopamine-induced lesion in planarian brain. J. Neurochem 2011, 119, 1217–1231. [Google Scholar]
- Haavik, J.; Toska, K. Tyrosine hydroxylase and Parkinson’s disease. Mol. Neurobiol 1998, 16, 285–309. [Google Scholar]
- Tabrez, S.; Jabir, N.R.; Shakil, S.; Greig, N.H.; Alam, Q.; Abuzenadah, A.M.; Damanhouri, G.A.; Kamal, M.A. A synopsis on the role of tyrosine hydroxylase in Parkinson’s disease. CNS Neurol. Dis. Drug Targets 2012, 11, 395–409. [Google Scholar]
- Ness, D.K.; Foley, G.L.; Villar, D.; Hansen, L.G. Effects of 3-iodo-l-tyrosine, a tyrosine hydroxylase inhibitor, on eye pigmentation and biogenic amines in the Planarian Dugesia dorotocephala. Fund. Appl. Toxicol 1996, 30, 153–161. [Google Scholar]
- Schapira, A.H.V.; Olanow, C.W. Rationale for the use of dopamine agonists as neuroprotective agents in Parkinson’s disease. Ann. Neurol 2003, 53, S149–S159. [Google Scholar]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol 1973, 22, 3099–3108. [Google Scholar]
- Palladini, G.; Ruggeri, S.; Stocchi, F.; de Pandis, M.F.; Venturini, G.; Margotta, V. A pharmacological study of cocaine activity in planaria. Comp. Biochem. Physiol. C 1996, 115, 41–45. [Google Scholar]
- Pagán, O.R.; Rowlands, A.L.; Urban, K.R. Toxicity and behavioral effects of dimethylsulfoxide in planaria. Neurosci. Lett 2006, 407, 274–278. [Google Scholar]
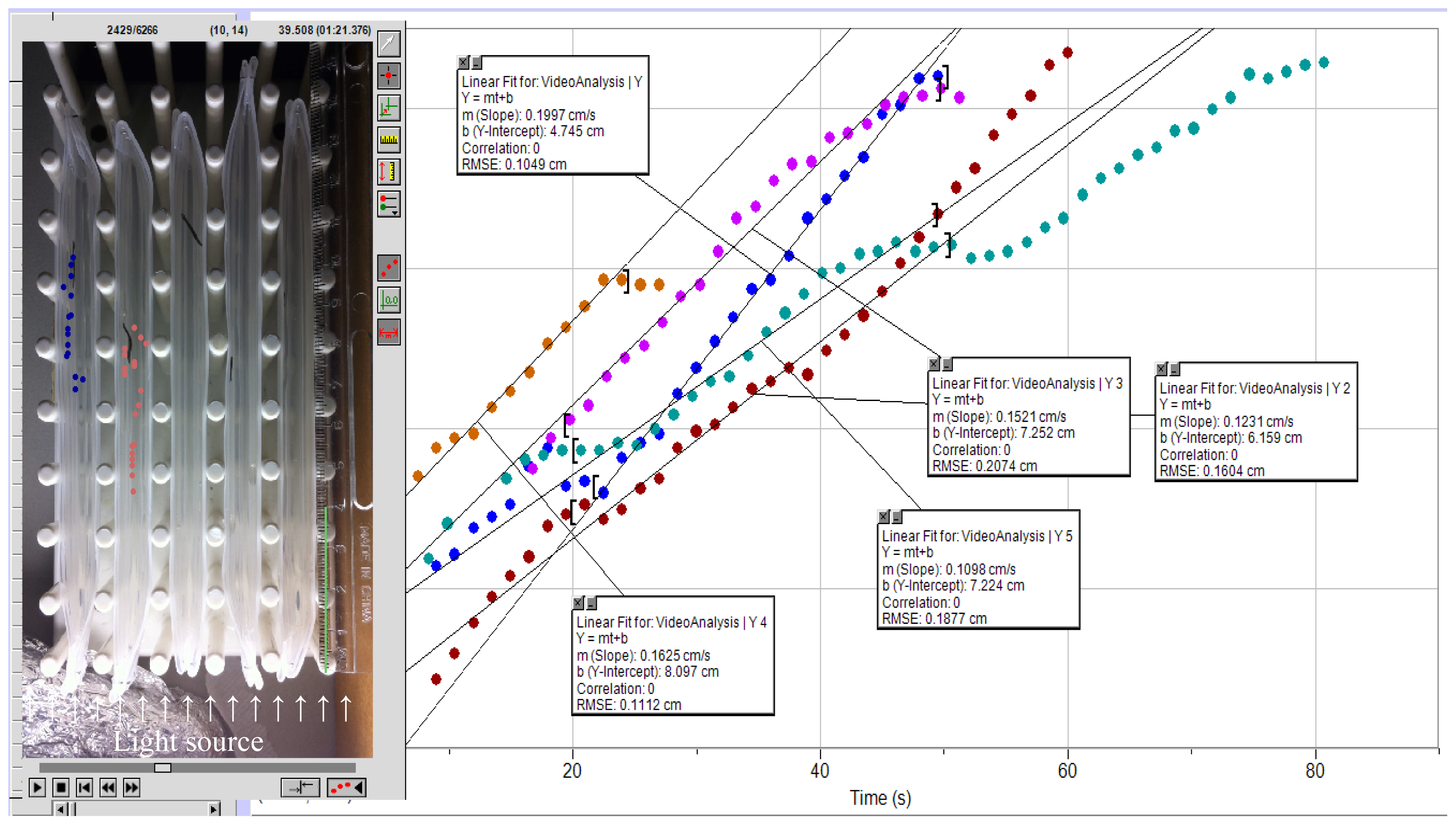
| MIT Concentration (mM) | Baseline b | 24-h exposure | 48-h exposure | 72-h exposure | 96-h exposure |
|---|---|---|---|---|---|
| 0 b | 12.4 ± 0.3 | 12.7 ± 0.4 | 11.9 ± 0.7 | 13.3 ± 0.6 | 11.8 ± 0.4 |
| 0.001 | 11.8 ± 0.1 | 11.1 ± 0.4 | 11.3 ± 1.2 | 11.5 ± 0.6 | 10.6 ± 0.4 |
| 0.1 | 12.2 ± 0.5 | 7.8 ± 0.6 | 10.3 ± 1.7 | 9.4 ± 1.7 | 8.4 ± 0.3 |
| 1 | 12.9 ± 1.1 | 4.8 ± 0.6 | 7.7 ± 2.9 | 7.3 ± 2.3 | 5.9 ± 1.8 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prokai, D.; Nguyen, T.; Kamrowski, K.; Chandra, A.; Talamantes, T.; Baxter, L.R.; Prokai, L. An Exploratory Evaluation of Tyrosine Hydroxylase Inhibition in Planaria as a Model for Parkinsonism. Int. J. Mol. Sci. 2013, 14, 23289-23296. https://doi.org/10.3390/ijms141223289
Prokai D, Nguyen T, Kamrowski K, Chandra A, Talamantes T, Baxter LR, Prokai L. An Exploratory Evaluation of Tyrosine Hydroxylase Inhibition in Planaria as a Model for Parkinsonism. International Journal of Molecular Sciences. 2013; 14(12):23289-23296. https://doi.org/10.3390/ijms141223289
Chicago/Turabian StyleProkai, David, Thinh Nguyen, Kurt Kamrowski, Ashwin Chandra, Tatjana Talamantes, Lewis R. Baxter, and Laszlo Prokai. 2013. "An Exploratory Evaluation of Tyrosine Hydroxylase Inhibition in Planaria as a Model for Parkinsonism" International Journal of Molecular Sciences 14, no. 12: 23289-23296. https://doi.org/10.3390/ijms141223289






