Synthesis and Antimicrobial Evaluation of Some Novel Bis-?,?-Unsaturated Ketones, Nicotinonitrile, 1,2-Dihydropyridine-3-carbonitrile, Fused Thieno[2,3-b]pyridine and Pyrazolo[3,4-b]pyridine Derivatives
Abstract
:1. Introduction
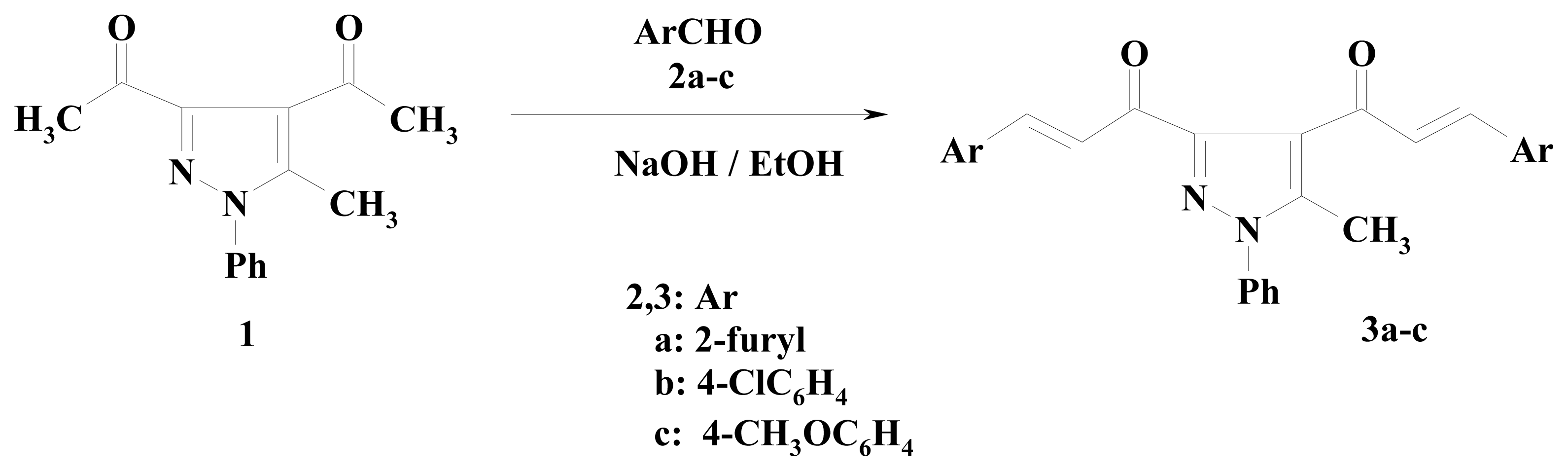
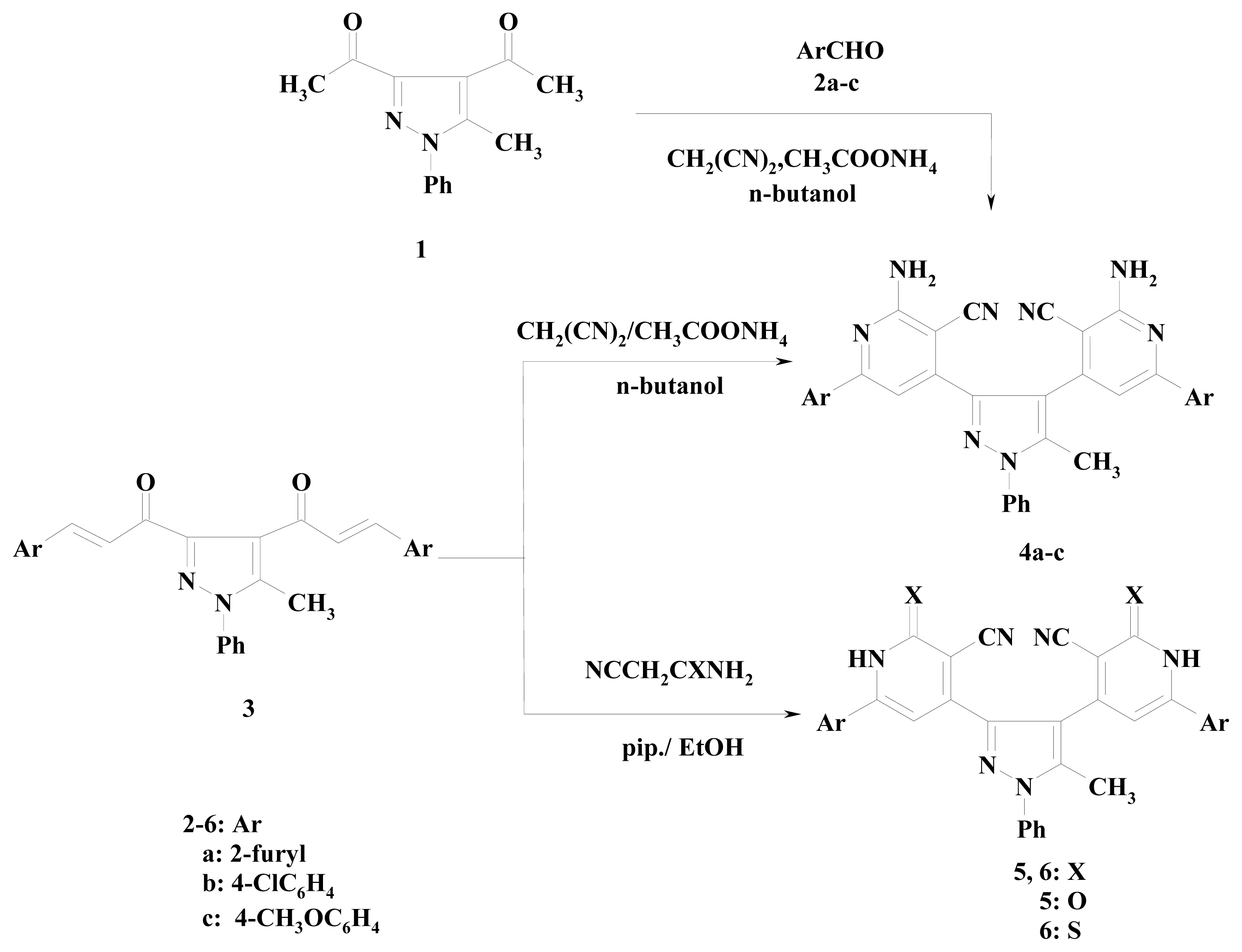
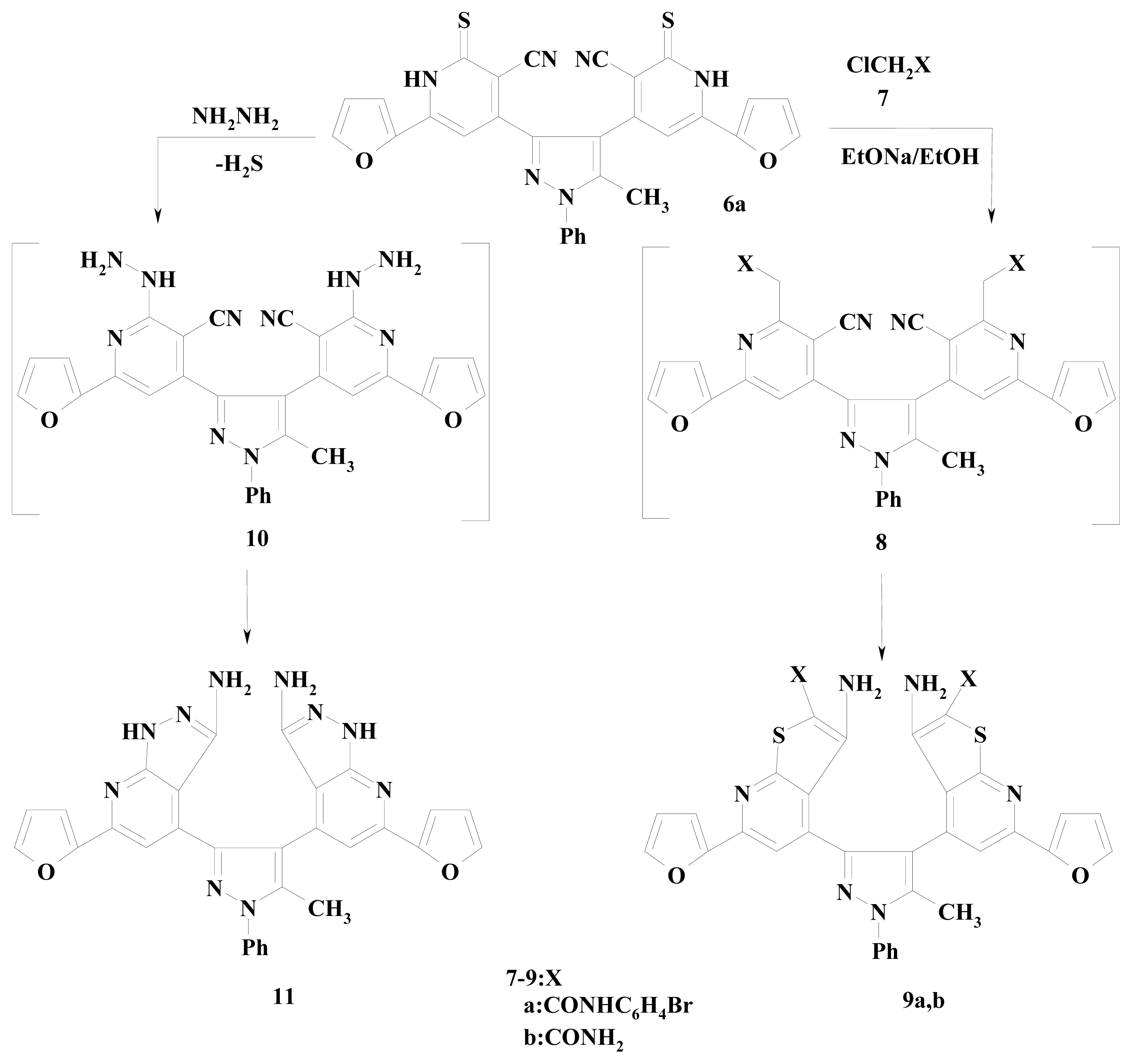
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Synthetic Procedures
3.2.1. 1,1′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[3-Aryl prop-2-en-1-one] (3a–c)
3.2.2. 1,1′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[3-(2-furyl)prop-2-en-1-one] (3a)
3.2.3. 1,1′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[3-(4-chlorophenyl) prop-2-en-1-one] (3b)
3.2.4. 1,1′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[3-(4-methoxyphenyl)prop-2-en-1-one] (3c)
3.2.5. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[2-amino-6-(aryl)nicotinonitrile] (4a–c)
3.2.6. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[2-amino-6-(2-furyl)nicotinonitrile] (4a)
3.2.7. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[2-amino-6-(4-chlorophenyl)nicotinonitrile] (4b)
3.2.8. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[2-amino-6-(4-methoxyphenyl)nicotinonitrile] (4c)
3.2.9. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[6-(2-aryl)-2-oxo-1,2-dihydropyridine-3-carbonitrile] (5a–c)
3.2.10. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[6-(2-furyl)-2-oxo-1,2-dihydropyridine-3-carbonitrile] (5a)
3.2.11. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[6-(4-chlorophenyl)-2-oxo-1,2-dihydropyridine-3-carbonitrile] (5b)
3.2.12. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[6-(4-methoxyphenyl)-2-oxo-1,2-dihydropyridine-3-carbonitrile] (5c)
3.2.13. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[6-(2-furyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile] (6a,b)
3.2.14. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[6-(2-furyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile] (6a)
3.2.15. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[6-(4-chlorophenyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile] (6b)
3.2.16. Synthesis of Compounds 9a,b
3.2.17. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[3-amino-N-(4-bromophenyl)-6-(2-furyl) thieno[2,3-b]pyridine-2-carboxamide] (9a)
3.2.18. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[3-amino-6-(2-furyl) thieno[2,3-b]pyridine-2-carboxamide] (9b)
3.2.19. 4,4′-(5-Methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis[6-(2-furyl)-1H-pyrazolo[3,4-b]pyridin-3-amine] (11)
3.3. Antimicrobial Evaluation
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Li, J.T.; Sun, M.X.; He, G.Y.; Xu, X.Y. Efficient and green synthesis of bis(indolyl)methanes catalyzed by ABS in aqueous media under ultrasound irradiation. Ultrason. Sonochem 2011, 18, 412–414. [Google Scholar]
- Wang, Z.; Zhao, C.; Zhao, D.; Li, C.; Ahang, J.; Wang, H. The preparation of substituted bithiophenyl aldehydes via the ring opening of dithieno[2,3-b:3′,2′-d]thiophene in the presence of n-BuLi. Tetrahedron 2010, 66, 2168–2174. [Google Scholar]
- Diana, P.; Carbone, A.; Barraja, P.; Kelter, G.; Fiebig, H.; Cirrincione, G. Synthesis and antitumor activity of 2,5-bis(3′-indolyl)-furans and 3,5-bis(3′-indolyl)-isoxazoles, nortopsentin analogues. Bioorg. Med. Chem 2010, 18, 4524–4529. [Google Scholar]
- Toyota, K.; Okada, K.; Katsuta, H.; Morita, N. Preparations of bis[2-(2-arylethynyl)-3-thienyl]arenes and bis[2-{2-(trimethylsilyl)ethynyl}-3-thienyl]arenes. Tetrahedron 2009, 65, 145–151. [Google Scholar]
- Todd, E.M.; Zimmerman, S.C. Bis-ureidodeazapterin (Bis-DeAP) as a general route to supra molecular star polymers. Tetrahedron 2008, 64, 8558–8570. [Google Scholar]
- Diana, P.; Carbone, A.; Barraja, P.; Montalbano, A.; Martorana, A.; Dattolo, G.; Gia, O.; Dalla Via, L.; Cirrincione, G. Synthesis and antitumor properties of 2,5-bis(3′-indolyl)thiophenes: Analogues of marine alkaloid nortopsentin. Bioorg. Med. Chem. Lett 2007, 17, 2342–2346. [Google Scholar]
- Blanco, G.; Quintela, J.M.; Peinador, C. Efficient one-pot preparation of bis(pyrazino-[2′,3′:4,5]thieno-[3,2-d]pyrimidin-4-yl)benzenes based on an aza—Wittig/mediated annulation strategy. Tetrahedron 2007, 63, 2034–2041. [Google Scholar]
- Promarak, V.; Punkvuang, A.; Jungsuttiwong, S.; Saengsuwan, S.; Sudyoadsuk, T.; Keawin, T. Synthesis, optical, electrochemical, and thermal properties of α,α′-bis(9,9-bis-n-hexylfluorenyl) substituted oligothiophenes. Tetrahedron Lett 2007, 48, 3661–3665. [Google Scholar]
- Awasthi, S.K.; Mishra, N.; Kumar, B.; Sharma, M.; Bhattacharya, A.; Mishra, L.C.; Bhasin, V.K. Potent antimalarial activity of newly synthesized substituted chalcone analogs in vitro. Med. Chem. Res 2009, 18, 407–420. [Google Scholar]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Sedek, L.; Paradysz, A.; Krol, W. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int. J. Mol. Sci 2010, 11, 1–13. [Google Scholar]
- Echeverria, C.; Santibanez, J.F.; Donoso-Tauda, O.; Escobar, C.A.; Tagle, R.R. Structural antitumoral activity relationships of synthetic chalcones. Int. J. Mol. Sci 2009, 10, 221–231. [Google Scholar]
- Ilango, K.; Valentina, P.; Saluja, G. Synthesis and in vitro anticancer activity of some substituted chalcones derivatives. Res. J. Pharm. Biol. Chem. Sci 2010, 1, 354–359. [Google Scholar]
- Neves, M.P.; Lima, R.T.; Choosang, K.; Pakkong, P.; Nascimento, M.S.J.; Vasconcelos, H.; Pinto, M.; Silva, A.M.S.; Cidade, H. Synthesis of a natural chalcone and its prenyl analogs-evaluation of tumor cell growth-inhibitory activities, and effects on cell cycle and apoptosis. Chem. Biodivers 2012, 9, 1133–1143. [Google Scholar]
- Zhang, X.W.; Zhao, D.H.; Quan, Y.C.; Sun, L.P.; Yin, X.M.; Guan, L.P. Synthesis and evaluation of anti-inflammatory activity of substituted chalcone derivatives. Med. Chem. Res 2010, 19, 403–412. [Google Scholar]
- Lunardi, F.; Guzela, M.; Rodrigues, A.T.; Corre, R.; Eger-Mangrich, I.; Steindel, M.; Grisard, E.C.; Assreuy, J.; Calixto, J.B.; Santos, A.R.S. Trypanocidal and leishmanicidal properties of substitution-containing chalcones. Antimicrob. Agents Chemother 2003, 47, 1449–1451. [Google Scholar]
- Bag, S.; Ramar, S.; Degani, M.S. Synthesis and biological evaluation of α,β-unsaturated ketone as potential antifungal agents. Med. Chem. Res 2009, 18, 309–316. [Google Scholar]
- Raghukumar, V.; Thirumalai, D.; Ramakrishnan, V.T.; Karunakarac, V.; Ramamurthy, P. Synthesis of nicotinonitrile derivatives as a new class of NLO materials. Tetrahedron 2003, 59, 3761–3768. [Google Scholar]
- Kanbara, T.; Koshida, K.; Sato, N.; Kuwajima, I.; Kubota, K.; Yamamoto, T. Preparation and properties of highly electron-accepting poly(pyrimidine-2,5-diyl). Chem. Lett 1992, 21, 583–586. [Google Scholar]
- Kotb, E.R.; El-Hashash, M.A.; Salama, M.A.; Kalf1, H.S.; Abdel Wahed, N.A.M. Synthesis and reactions of some novel nicotinonitrile derivatives for anticancer and antimicrobial evaluation. Acta Chim. Slov 2009, 56, 908–919. [Google Scholar]
- Saini, A.; Kumar, S.; Sandhu, J.S. Hantzsch reaction: Recent advances in Hantzch 1,4-dihyropyridines. J. Sci. Ind. Res 2008, 67, 95–111. [Google Scholar]
- Schramm, M.; Thomas, G.; Franckowiak, G. Novel dihydropyridines with positive iontropic action through of Ca2+ channels. Nature 1983, 303, 535–537. [Google Scholar]
- Nakayama, H.; Kasoaka, Y. Chemical identification of binding sites of calcium channel antagonists. Heterocycles 1996, 42, 901–909. [Google Scholar]
- Desai, B.; Sureja, D.; Naliapara, Y.; Shah, A.; Saxena, A.K. Synthesis and QSAR studies of 4-substituted phenyl-2,6-dihydropyridines as potential anti tubercular agents. Bioorg. Med. Chem 2001, 9, 1993–1998. [Google Scholar]
- Boer, R.; Gekeler, V. Chemsensitizers in tumor therapy: New compounds promise better efficacy. Drugs Fut 1995, 20, 499–509. [Google Scholar]
- Bashandy, M.S.; Al-Said, M.S.; Al-Qasoumi, S.I.; Ghorab, M.M. Design and synthesis of some novel hydrazide, 1,2-dihydropyridine, chromene derivatives carrying biologically active sulfone moieties with potential anticancer activity. Arzneimittelforschung 2011, 61, 521–526. [Google Scholar]
- Al-Said, M.S.; Bashandy, M.S.; Al-Qasoumi, S.I.; Ghorab, M.M. Anti-breast cancer activity of some novel 1,2-dihydropyridine, thiophene and thiazole derivatives. Eur. J. Med. Chem 2011, 46, 137–141. [Google Scholar]
- Abdel-Fattah, M.A.; El-Naggar, M.A.M.; Rashied, R.M.H.; Gary, B.D.; Piazza, G.A.; Abadi, A.H. Four-component synthesis of 1,2-dihydropyridine derivatives and their evaluation as anticancer agents. Med. Chem 2012, 8, 392–400. [Google Scholar]
- Carrión, M.D.; Lopez Cara, L.C.; Camacho, E.V.; Tapias, M.; Escames, G.; Acuña-Castroviejo, D.; Espinosa, A.; Gallo, M.A.; Entrena, A. Pyrazoles and pyrazolines as neural and inducible nitric oxide synthase (nNOS and iNOS) potential inhibitors (III). Eur. J. Med. Chem 2008, 43, 2579–2591. [Google Scholar]
- Gökhan-Kelekçi, N.; Koyunoğlu, S.; Yabanoğlu, S.; Yelekçi, K.; Özgen, Ö.; Uçar, G.; Erol, K.; Kendi, E.; Ye-ilada, A. New pyrazoline bearing 4(3H)-quinazolinone inhibitors of monoamine oxidase: Synthesis and biological evaluation and structural determinants of MAO-A and MAO-B selectivity. Bioorg. Med. Chem. 2009, 17, 675–689. [Google Scholar]
- Yang, J.F.; Cao, H.; Liu, H.; Li, B.Q.; Ma, Y.M. Synthesis and bioactivity of novel bis-heterocyclic compounds containing pyrazole and oxadiazoline. J. Chin. Chem. Soc 2011, 58, 369–375. [Google Scholar]
- Abid, M.; Bhat, A.R.; Athar, F.; Azam, A. Synthesis, spectral studies and antiamoebic activity of new 1-N-substituted thiocarbamoyl-3-phenyl-2-pyrazolines. Eur. J. Med. Chem 2009, 44, 417–425. [Google Scholar]
- Farag, A.M.; Mayhoub, A.S.; Barakat, S.E.; Bayomi, A.H. Regioselective synthesis and antitumor screening of some novel N-phenylpyrazole derivatives. Bioorg. Med. Chem 2008, 16, 881–889. [Google Scholar]
- Farag, A.M.; Mayhoub, A.S.; Barakat, S.E.; Bayomi, A.H. Synthesis of new N-phenylpyrazole derivatives with potent antimicrobial activity. Bioorg. Med. Chem 2008, 16, 4569–4578. [Google Scholar]
- Hui, Y.; Ptak, R.; Paulman, R.; Pallansch, M.; Changa, C.W.T. Synthesis of novel guanidine incorporated aminoglycosides, guanidinopyranmycins. Tetrahedron Lett 2002, 43, 9255–9257. [Google Scholar]
- Witherington, J.; Bordas, V.; Gaiba, A.; Naylor, A.; Rawlings, A.D.; Slingsby, B.P.; Smith, D.G.; Takle, A.K.; Ward, R.W. 6-Heteroaryl-pyrazolo[3,4-b]pyridines: Potent and selective inhibitors of glycogen synthase kinase-3 (GSK-3). Bioorg. Med. Chem. Lett 2003, 13, 3059–3062. [Google Scholar]
- Fahmy, S.M.; Mohareb, R.M. Cyanothioacetamide in heterocyclic synthesis: A novel synthesis of 2-pyridothione derivatives. Tetrahedron 1986, 42, 687–690. [Google Scholar]
- Schmidt, U.; Kubitzek, H. Comparative kinetic studies on the activation of the methyl group by oxygen and sulfur containing groups. Chem. Bericht 1960, 93, 1559–1571. [Google Scholar]
- Salem, M.A.; Thabet, H.K.; Ismail, M.A.; Ammar, Y.A. 2N-Aryl 2-cyanothioacetamide intermediates in heterocyclic synthesis: Synthesis and antimicrobial evaluation of 3-cyano-2(1H)-pyridinethione, chromene-3-carbothioamide and chromeno[3,4-c]pyridinethione derivatives. Chem. Sci. J 2011, 36, 1–11. [Google Scholar]
- Shawali, A.S.; Haboub, A.J.M. Bis-enaminones as precursors for synthesis of novel 3,4-bis(heteroaryl) pyrazoles and 3,6-bis-(heteroaryl)-pyrazolo[3,4-d]pyridazines. J. Chem. Res 2011, 35, 341–345. [Google Scholar]
- Fahmi, A.A. Synthesis of some 2H-pyrazolo[3,4-d]pyridazines. Int. J. Chem 1995, 6, 1–4. [Google Scholar]
- Tewari, R.S.; Parihar, P. Studies on nitrile imines: Synthesis of pyrazoles using active methylene compounds. Indian J. Chem 1980, 19, 217–218. [Google Scholar]
- Abdel-Aziz, H.A.; Al-Rashood, K.A.; ElTahirb, K.E.; Ibrahim, H.S. Microwave-assisted synthesis of novel 3,4-bis-chalcone-N-arylpyrazoles and their anti-inflammatory activity. J. Chin. Chem. Soc 2011, 58, 863–868. [Google Scholar]
- Esmail, R.; Kurzer, F. Heterocyclic compounds from urea derivatives. Part XXIII. Thiobenzoylated thiocarbonohydrazides and their cyclisation. J. Chem. Soc 1975, 18, 1787–1791. [Google Scholar]
- Muanz, D.N.; Kim, B.W.; Euler, K.L.; Williams, L. Antibacterial and antifungal activities of nine medicinal plants from Zaire. Int. J. Pharmacog 1994, 32, 337–345. [Google Scholar]
- Harborne, J.B.; Williams, C.A. A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry 1994, 37, 19–42. [Google Scholar]
| Compound No. | Inhibition Zone Diameter (cm) | |||
|---|---|---|---|---|
| Gram (−) | Gram (+) | Fungi | ||
| (EC) | (SA) | (AF) | (CA) | |
| 3a | 10 | 0.0 | 0.0 | 0.0 |
| 3b | 0.0 | 0.0 | 0.0 | 0.0 |
| 3c | 0.0 | 0.0 | 0.0 | 0.0 |
| 4a | 0.0 | 0.0 | 0.0 | 0.0 |
| 4b | 0.0 | 12 | 0.0 | 0.0 |
| 4c | 16 | 16 | 0.0 | 0.0 |
| 5a | 0.0 | 10 | 0.0 | 0.0 |
| 5c | 0.0 | 0.0 | 0.0 | 0.0 |
| 6a | 0.0 | 0.0 | 0.0 | 0.0 |
| 6b | 0.0 | 0.0 | 0.0 | 0.0 |
| 9a | 13 | 14 | 0.0 | 0.0 |
| Tetracycline | 30 | 29 | ||
| Diflucan | 18 | 21 | ||
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Altalbawy, F.M.A. Synthesis and Antimicrobial Evaluation of Some Novel Bis-?,?-Unsaturated Ketones, Nicotinonitrile, 1,2-Dihydropyridine-3-carbonitrile, Fused Thieno[2,3-b]pyridine and Pyrazolo[3,4-b]pyridine Derivatives. Int. J. Mol. Sci. 2013, 14, 2967-2979. https://doi.org/10.3390/ijms14022967
Altalbawy FMA. Synthesis and Antimicrobial Evaluation of Some Novel Bis-?,?-Unsaturated Ketones, Nicotinonitrile, 1,2-Dihydropyridine-3-carbonitrile, Fused Thieno[2,3-b]pyridine and Pyrazolo[3,4-b]pyridine Derivatives. International Journal of Molecular Sciences. 2013; 14(2):2967-2979. https://doi.org/10.3390/ijms14022967
Chicago/Turabian StyleAltalbawy, Farag M. A. 2013. "Synthesis and Antimicrobial Evaluation of Some Novel Bis-?,?-Unsaturated Ketones, Nicotinonitrile, 1,2-Dihydropyridine-3-carbonitrile, Fused Thieno[2,3-b]pyridine and Pyrazolo[3,4-b]pyridine Derivatives" International Journal of Molecular Sciences 14, no. 2: 2967-2979. https://doi.org/10.3390/ijms14022967
APA StyleAltalbawy, F. M. A. (2013). Synthesis and Antimicrobial Evaluation of Some Novel Bis-?,?-Unsaturated Ketones, Nicotinonitrile, 1,2-Dihydropyridine-3-carbonitrile, Fused Thieno[2,3-b]pyridine and Pyrazolo[3,4-b]pyridine Derivatives. International Journal of Molecular Sciences, 14(2), 2967-2979. https://doi.org/10.3390/ijms14022967





