Non-Enzymatic Modification of Aminophospholipids by Carbonyl-Amine Reactions
Abstract
:1. Introduction
2. Membrane Unsaturation and Lipid Peroxidation
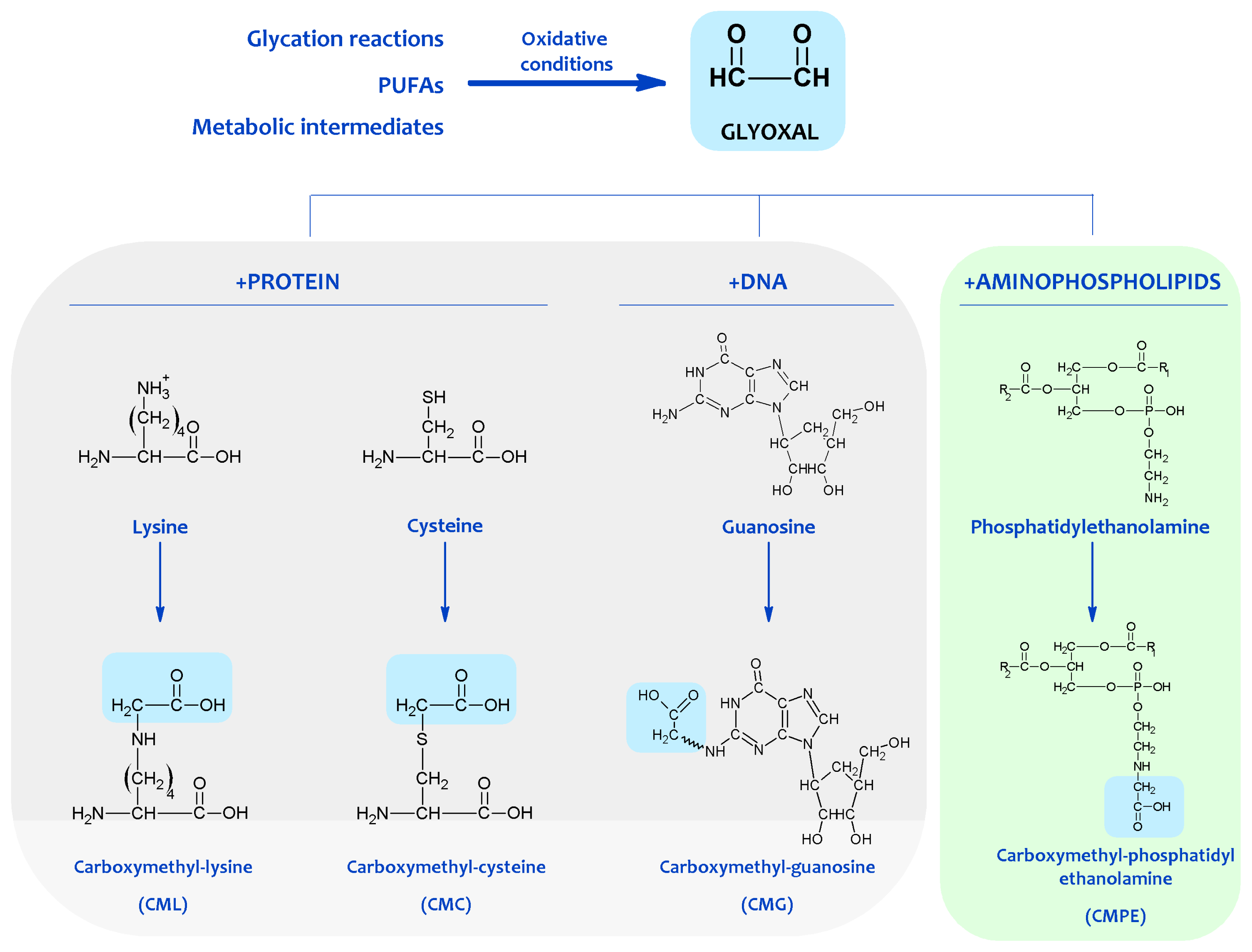
3. Non-Enzymatic Modification of Cellular Components: The Maillard Reaction-Derived Molecular Damage
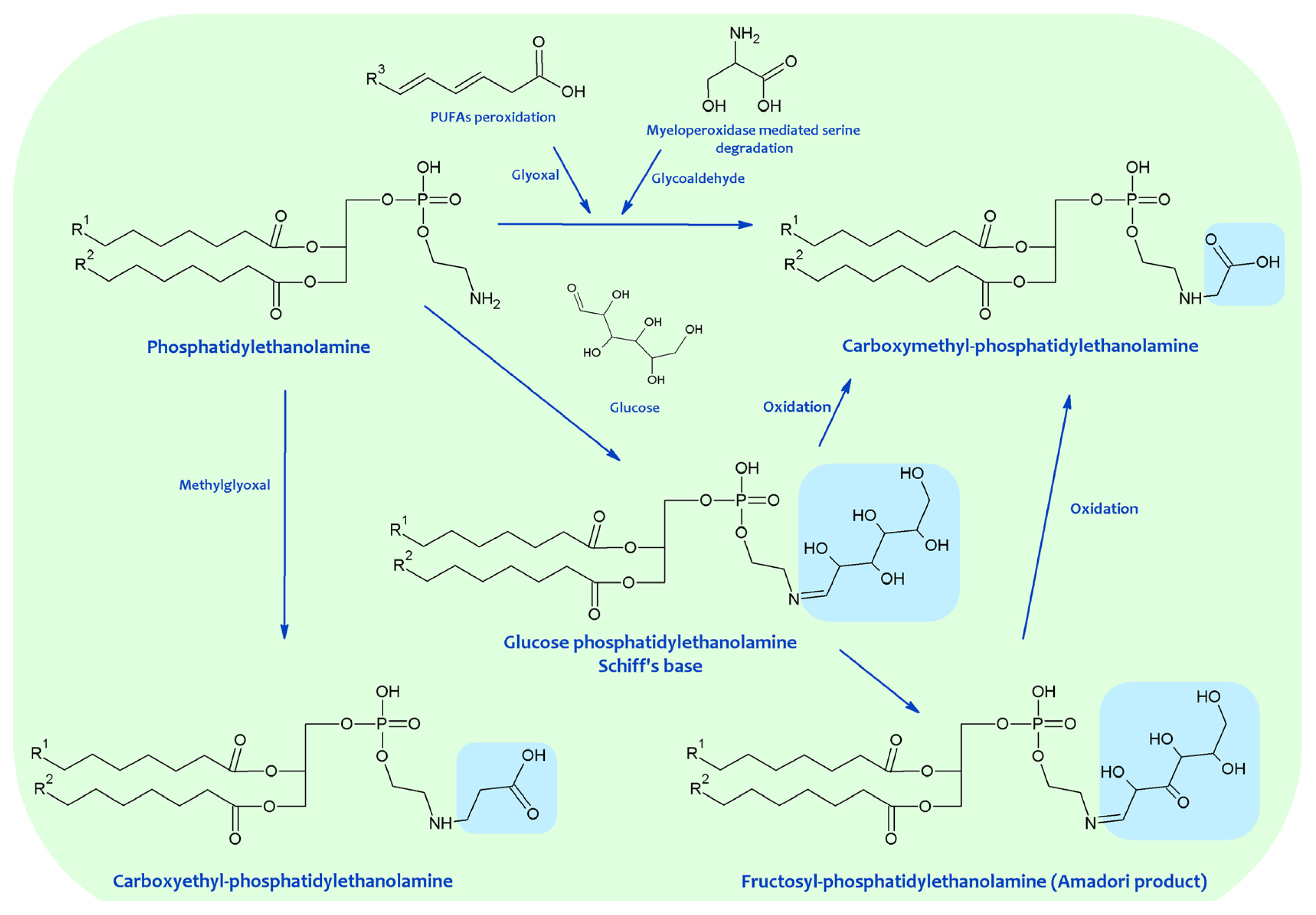
4. Chemical Modification of Aminophospholipids by Carbonyl-Amine Reactions
5. Biological Significance of MRPs in Aminophospholipids
6. Physiological Significance of MRPs in Lipids
7. Significance of MRPs in Aminophospholipids in Aging and Pathological Conditions
8. Summary
Acknowledgments
References
- Yeagle, P. The Membranes of Cells; Academic Press: San Diego, CA, USA, 1993; pp. 1–349. [Google Scholar]
- Vance, D.E.; Vance, J.E. Biochemistry of Lipids, Lipoproteins and Membranes; Elsevier Science BV: Amsterdam, The Netherlands, 1996; pp. 1–553. [Google Scholar]
- Vereb, G.; Szollosi, J.; Matko, J.; Nagy, P.; Farkas, T.; Vigh, L.; Matyus, L.; Waldmann, T.A.; Damjanovich, S. Dynamic, yet structured: The cell membrane three decades after the Singer-Nicolson model. Proc. Natl. Acad. Sci. USA 2003, 100, 8053–8058. [Google Scholar]
- Ikeda, M.; Kihara, A.; Igarashi, Y. Lipid asymmetry of the eukaryotic plasma membrana: Functions and related enzymes. Biol. Pharm. Bull 2006, 29, 1542–1546. [Google Scholar]
- Lenoir, G; Williamson, P.; Holthuis, J.C.M. On the origin of lipid asymmetry: The flip side of ion transport. Curr. Opin. Chem. Biol. 2007, 11, 654–661. [Google Scholar]
- Dowhan, W. Molecular basis for membrane phospholipid diversity: Why are there so many lipids? Ann. Rev. Biochem 1997, 66, 199–232. [Google Scholar]
- Van Meer, G. Cellular lipidomics. EMBO J 2005, 24, 3159–3165. [Google Scholar]
- Portero-Otín, M.; Bellmunt, M.J.; Ruiz, M.C.; Barja, G.; Pamplona, R. Correlation of fatty acid unsaturation of the major liver mitochondrial phospholipid classes in mammals to their maximum life span potential. Lipids 2001, 36, 491–498. [Google Scholar]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol 2008, 9, 112–124. [Google Scholar]
- Hermansson, M.; Hokynar, K.; Somerharju, P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid Res 2011, 50, 240–257. [Google Scholar]
- Schroit, A.J.; Zwaal, R.F.A. Transbilayer movement of phospholipids in red cell and platelet membranes. Biochim. Biophys. Acta 1991, 1071, 313–329. [Google Scholar]
- Balasubramanian, K.; Schroit, A.J. Aminophospholipid asymmetry: A matter of lifeand death. Ann. Rev. Physiol 2003, 65, 701–734. [Google Scholar]
- Wallis, J.G.; Watts, J.L.; Browse, J. Polyunsaturated fatty acid synthesis: What will they think of next? Trends Biochem. Sci 2002, 27, 467–473. [Google Scholar]
- Pamplona, R. Membrane phospholipids, lipoxidative damage and molecular integrity: A causal role in aging and longevity. Biochim. Biophys. Acta 2008, 1777, 1249–1262. [Google Scholar]
- Hulbert, A.J.; Pamplona, R.; Buffenstein, R.; Buttemer, W.A. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol. Rev 2007, 87, 1175–1213. [Google Scholar]
- Moller, M.; Botti, H.; Batthyany, C.; Rubbo, H.; Radi, R.; Denicola, A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J. Biol. Chem 2005, 280, 8850–8854. [Google Scholar]
- Gamliel, A.; Afri, M.; Frimer, A.A. Determining radical penetration of lipid bilayers with new lipophilic spin traps. Free Radical Biol. Med 2008, 44, 1394–1405. [Google Scholar]
- Holman, R.T. Autoxidation of Fats and Related Substances. In Progress in Chemistry of Fats and Other Lipids; Holman, R.T., Lundberg, W.O., Malkin, T., Eds.; Pergamon Press: London, UK, 1954; pp. 51–98. [Google Scholar]
- Bielski, B.H.; Arudi, R.L.; Sutherland, M.W. A study of the reactivity of HO2/O2− with unsaturated fatty acids. J. Biol. Chem 1983, 258, 4759–4761. [Google Scholar]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol. Med 1991, 11, 81–128. [Google Scholar]
- Aldini, G.; Dalle-Donne, I.; Facino, R.M.; Milzani, A.; Carini, M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev 2007, 27, 817–868. [Google Scholar]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar]
- Pamplona, R. Advanced lipoxidation end-products. Chem.-Biol. Interact 2011, 192, 14–20. [Google Scholar]
- Terman, A.; Brunk, U.T. Lipofuscin. Int. J. Biochem. Cell Biol 2004, 36, 1400–1404. [Google Scholar]
- West, J.D.; Marnett, L.J. Endogenous reactive intermediates as modulators of cell signalling and cell death. Chem. Res. Toxicol 2006, 19, 173–194. [Google Scholar]
- Baynes, J.W.; Monnier, V.M. The Maillard Reaction in Aging, Diabetes and Nutrition; Liss: New York, NY, USA, 1989. [Google Scholar]
- Portero-Otin, M.; Pamplona, R. Is Endogenous Oxidative Protein Damage Envolved in the Aging Process? In Protein Oxidation and Disease; Pietzsch, J., Ed.; Research Signpost: Kerala, India, 2006; pp. 91–142. [Google Scholar]
- Baynes, J.W. The Role of Oxidation in the Maillard Reaction in vivo. In The Maillard Reaction: Consequences for the Chemical and Life Sciences; Ika, R., Ed.; John Wiley & Sons: Chichester, UK, 1996; pp. 55–72. [Google Scholar]
- Corliss, G.A.; Dugan, L.R. Phospholipid oxidation in emulsions. Lipids 1970, 5, 846–853. [Google Scholar]
- Popjak, G.; LeBreton, E. Biochemical Problems of Lipids; Interscience Publishers Inc.: New York, NY, USA, 1956; p. 81. [Google Scholar]
- Dillard, C.J.; Tappel, A.L. Fluorescent products from reaction of peroxidizing polyunsaturated fatty acids with phosphatidyl ethanolamine and phenylalanine. Lipids 1973, 8, 183–189. [Google Scholar]
- Bidlack, W.R.; Tappel, A.L. Fluorescent products of phospholipids during lipid peroxidation. Lipids 1973, 8, 203–207. [Google Scholar]
- Shimasaki, H.; Ueta, N.; Mowri, H.-O.; Inoue, K. Formation of age pigment-like fluorescent substances during peroxidation of lipids in model membranes. Biochim. Biophys. Acta 1984, 792, 123–129. [Google Scholar]
- Tsuchida, M.; Miura, T.; Aibara, K. Lipofucsin and lipofucsin-like substances. Chem. Phys. Lipids 1987, 44, 297–325. [Google Scholar]
- Jain, S.K.; Hochstein, P. Polymerization of membrane components in aging red blood cells. Biochem. Biophys. Res. Commun 1980, 92, 247–254. [Google Scholar]
- Jain, S.K.; Yip, R.; Hoesch, R.M.; Pramanik, A.K.; Dallman, P.R.; Shohet, S.B. Evidence of peroxidative damage to the erythrocyte membrane in iron deficiency. Am. J. Clin. Nutr 1983, 37, 26–30. [Google Scholar]
- Jain, S.K.; Shohet, S.B. A novel phospholipid in irreversibly sickled cells: Evidence for in vivo peroxidative membrane damage in sickle cell disease. Blood 1984, 63, 362–367. [Google Scholar]
- Jain, S.K. The accumulation of malonyldialdehyde, a product of fatty acid peroxidation, can disturb aminophospholipid organization in the membrane bilayer of human erythrocytes. J. Biol. Chem 1984, 259, 3391–3394. [Google Scholar]
- Jain, S.K. In vivo externalization of phosphatidylserine and phosphatidylethanolamine in the membrane bilayer and hypercoagulability by the lipid peroxidation of erythrocytes in rats. J. Clin. Invest 1985, 76, 281–286. [Google Scholar]
- Jain, S.K. Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes. Biochim. Biophys. Acta 1988, 937, 205–210. [Google Scholar]
- Jain, S.K.; McVie, R.; Duett, J.; Herbst, J.J. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 1989, 38, 1539–1543. [Google Scholar]
- Jain, S.K. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J. Biol. Chem 1989, 264, 21340–21345. [Google Scholar]
- Bhuyan, K.C.; Master, R.W.; Coles, R.S.; Bhuyan, D.K. Molecular mechanisms of cataractogenesis: IV. Evidence of phospholipid.malondialdehyde adduct in human senile cataract. Mech. Ageing Dev 1986, 34, 289–296. [Google Scholar]
- Bhuyan, D.K.; Master, R.W.; Bhuyan, K.C. Crosslinking of aminophospholipids in cellular membranes of lens by oxidative stress in vitro. Biochim. Biophys. Acta 1996, 1285, 21–28. [Google Scholar]
- Esterbauer, H.; Koller, E.; Slee, R.G.; Koster, J.F. Possible involvement of the lipid-peroxidation product 4-hydroxynonenal in the formation of fluorescent chromolipids. Biochem. J 1986, 239, 405–409. [Google Scholar]
- Guichardant, M.; Taibi-Tronche, P.; Fay, L.B.; Lagarde, M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic. Biol. Med 1998, 25, 1049–1056. [Google Scholar]
- Dillard, C.J.; Tappel, A.L. Fluorescent products of lipid peroxidation of mitochondria and microsomes. Lipids 1971, 6, 715–721. [Google Scholar]
- Koster, J.F.; Slee, R.G. Lipid peroxidation of rat liver microsomes. Biochim. Biophys. Acta 1980, 620, 489–499. [Google Scholar]
- Kikugawa, K.; Ido, Y. Studies on peroxidized lipids. V. Formation and characterization of 1,4-dihydropyridine-3,5-dicarbaldehydes as model of fluorescent components in lipofuscin. Lipids 1984, 19, 600–608. [Google Scholar]
- Hadley, M.; Draper, H.H. Identification of N-(2-propenal)serine as a urinary metabolite of malondialdehyde. FASEB J 1988, 2, 138–140. [Google Scholar]
- Hadley, M.; Draper, H.H. Identification of N-(2-propenal)ethanolamine as a urinary metabolite of malondialdehyde. Free Radical Biol. Med 1989, 6, 49–52. [Google Scholar]
- Wang, J.-Y.; Wang, Z.-Y.; Kouyama, T.; Shibata, T.; Ueki, T. Significance of amino groups of phosphatidylethanolamine in phospholipid peroxidation of mixed liposomes. Chem. Phys. Lipids 1994, 71, 197–203. [Google Scholar]
- Ravandi, A.; Kuksis, A.; Marai, L.; Myher, J.J. Preparation and characterization of glucosylated aminoglycerophospholipids. Lipids 1995, 30, 885–891. [Google Scholar]
- Requena, J.R.; Ahmed, M.U.; Fountain, C.W.; Degenhardt, T.P.; Reddy, S.; Perez, C.; Lyons, T.J.; Jenkins, A.J.; Baynes, J.W.; Thorpe, S.R. Carboxymethylethanolamine, a biomarker of phospholipid modification during the Maillard reaction in vivo. J. Biol. Chem 1997, 272, 17473–17479. [Google Scholar]
- Lederer, M.O.; Dreisbusch, C.M.; Bundschuh, R.M. Amadori products from model reactions of d-glucose with phosphatidyl ethanolamine. Independent synthesis and identification of 1-deoxy-1-(2-hydroxyethylamino)-d-fructose derivatives. Carbohyd. Res 1997, 301, 111–121. [Google Scholar]
- Lertsiri, S.; Shiraishi, M.; Miyazawa, T. Identification of deoxy-D-fructosyl phosphatidyl ethanolamine as a non-enzymic glycation product of phosphatidyl ethanolamine and its occurrence in human blood plasma and red blood cells. Biosci. Biotechnol. Biochem 1998, 62, 893–901. [Google Scholar]
- Argirov, O.K.; Kerina, I.I.; Uzunova, J.I.; Argirova, M.D. Modeling of Protein and Aminophospholipid Glycation Using Low Molecular Weight Analogs. A Comparative Study. In The Maillard Reaction in Foods and Medicine; O’brien, J., Nursten, H.E., Crabbe, M.J.C., Ames, J.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 245–249. [Google Scholar]
- Fountain, W.C.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Smyth, B.; Baynes, J.W.; Thorpe, S.R. Quantification of N-(Glucitol)ethanolamine and N-(Carboxymethyl)serine: Two products of nonenzymatic modification of aminophospholipids formed in vivo. Anal. Biochem 1999, 272, 48–55. [Google Scholar]
- Heller, J.I.; Crowley, J.R.; Hazen, S.L.; Salvay, D.M.; Wagner, P.; Pennathur, S.; Heinecke, J.W. p-Hydroxyphenylacetaldehyde, an aldehyde generated by myeloperoxidase, modififes phospholipid amino groups of low density lipoprotein in human atherosclerotic intima. J. Biol. Chem 2000, 275, 9957–9962. [Google Scholar]
- Lederer, M.O.; Baumann, M. Formation of a phospholipid-linked pyrrolecarbaldehyde from model reactions of d-glucose and 3-deoxyglucosone with phosphatidyl ethanolamine. Bioorgan. Med. Chem 2000, 8, 115–121. [Google Scholar]
- Utzmann, C.M.; Lederer, M.O. Independent synthesis of aminophospholipid-linked Maillard products. Carbohyd. Res 2000, 325, 157–168. [Google Scholar]
- Zamora, R.; Hidalgo, F.J. Phosphatidylethanolamine modification by oxidative stress product 4,5(E)-epoxy-2(E)-heptenal. Chem. Res. Toxicol 2003, 16, 1632–1641. [Google Scholar]
- Tsuji, K.; Kawai, Y.; Kato, Y.; Osawa, T. Formation of N-(hexanoyl)ethanolamine, a novel phosphatidylethanolamine adduct, during the oxidation of erythrocyte membrane and low-density lipoprotein. Biochem. Biophys. Res. Commun 2003, 306, 706–711. [Google Scholar]
- Bacot, S.; Bernoud-Hubac, N.; Baddas, N.; Chantegrel, B.; Deshayes, C.; Doutheau, A.; Lagarde, M.; Guichardant, M. Covalent binding of hydroxyl-alkenals 4-HDDE, 4-HHE, and 4-HNE to ethanolamine phospholipid subclasses. J. Lipid Res 2003, 44, 917–926. [Google Scholar]
- Bernoud-Hubac, N.; Fay, L.B.; Armarnath, V.; Guichardant, M.; Bacot, S.; Davies, S.S.; Jackson Roberts, L., II; Lagarde, M. Covalent binding of isoketals to ethanolamine phospholipids. Free Radical Biol. Med. 2004, 37, 1604–1611. [Google Scholar]
- Stadelmann-Ingrand, S.; Pontcharraud, R.; Fauconneau, B. Evidence for the reactivity of fatty aldehydes released from oxidized plasmalogens with phosphatidylethanolamine to form Schiff base adducts in rat brain homogenates. Chem. Phys. Lipids 2004, 131, 93–105. [Google Scholar]
- Higuchi, O.; Nakagawa, K.; Tsuzuki, T.; Suzuki, T.; Oikawa, S.; Miyazawa, T. Aminophospholipid glycation and its inhibitor screening system: A new role of pyridoxal 5′-phosphate as the inhibitor. J. Lipid Res 2006, 47, 964–974. [Google Scholar]
- Miyazawa, T.; Ibusuki, D.; Yamashita, S.; Nakagawa, K. Analysis of amadori-glycated phosphatidylethanolamine in the plasma of healthy subjects and diabetic patients by liquid chromatography-tandem mass spectrometry. Ann. N. Y. Acad. Sci 2008, 1126, 291–294. [Google Scholar]
- Aguilar-Hernandez, M.; Mendez, J.D. In vitro glycation of brain aminophospholipids by acetoacetate and its inhibition by urea. Biomed. Pharmacother 2007, 61, 693–697. [Google Scholar]
- Solis-Calero, C.; Ortega-Castro, J.; Muñoz, F. Reactivity of a phospholipid monolayer model under periodic boundary conditions: A density functional theory study of the Schiff base formation between phosphatidylethanolamine and acetaldehyde. J. Phys. Chem 2010, 114, 15879–15885. [Google Scholar]
- Guo, L.; Chen, Z.; Amarnath, V.; Davies, S.S. identification of novel bioactive aldehyde-modified phosphatidylethanolamines formed by lipid peroxidation. Free Radic. Biol. Med 2012, 53, 1226–1238. [Google Scholar]
- Bucala, R.; Makita, Z.; Koschinsky, T.; Cerami, A.; Vlassara, H. Lipid advanced glycosylation: Pathway for lipid oxidation in vivo. Proc. Natl. Acad. Sci. USA 1993, 90, 6434–6438. [Google Scholar]
- Pamplona, R.; Bellmunt, M.J.; Portero-Otin, M.; Riba, D.; Prat, J. Chromatographic evidence for Amadori product formation in rat liver aminophospholipids. Life Sci 1995, 57, 873–879. [Google Scholar]
- Pamplona, R.; Requena, J.R.; Portero-Otin, M.; Prat, J.; Thorpe, S.R.; Bellmunt, M.J. Carboxymethylated phosphatidylethanolamine in mitochondrial membranes of mammals. Evidence for intracellular lipid glycoxidation. Eur. J. Biochem 1998, 225, 685–689. [Google Scholar]
- Requena, J.R.; Ahmed, M.U.; Reddy, S.; Fountain, C.W.; Degenhardt, T.P.; Jenkins, A.J.; Smyth, B.; Lyons, T.J.; Thorpe, S.R. Detection of AGE-Lipids in vivo: Glycation and Carboxymethylation of Aminophospholipids in Red Cell Membranes. In The Maillard Reaction in Foods and Medicine; O’brien, J., Nursten, H.E., Crabbe, M.J.C., Ames, J.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 363–368. [Google Scholar]
- Oak, J.-H.; Nakagawa, K.; Miyazawa, T. UV analysis of Amadori-glycated phosphatidylethanolamine in foods and biological samples. J. Lipid Res 2002, 43, 523–529. [Google Scholar]
- Ravandi, A.; Kuksis, A.; Marai, L.; Myher, J.J.; Steiner, G.; Lewisa, G.; Kamido, H. Isolation and identification of glycated aminophospholipids from red cells and plasma of diabetic blood. FEBS Lett 1996, 381, 77–81. [Google Scholar]
- Ravandi, A.; Kuksis, A.; Shaikh, N.; Jackowski, G. Preparation of Schiff base adducts of phosphatidylcholine core aldehydes and aminophospholipids, amino acids, and myoglobin. Lipids 1997, 32, 989–1001. [Google Scholar]
- Ravandi, A.; Kuksis, A.; Shaikh, N.A. Glycated phosphatidylethanolamine promotes macrophage uptake of low density lipoprotein and accumulation of cholesteryl esters and triacylglycerols. J. Biol. Chem 1999, 274, 16494–16500. [Google Scholar]
- Reddy, S.; Bichler, J.; Wells-Knecht, K.J.; Thorpe, S.R.; Baynes, J.W. Ne-(Carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry 1995, 34, 10872–10878. [Google Scholar]
- Jenkins, A.; Lyons, T.J.; Smyth, B.; Requena, J.R.; Fountain, C.W.; Hermayer, K.L.; Phillips, K.D.; King, L.P.; Baynes, J.W.; Thorpe, S.R. Glycoxidation and lipoxidation products in red blood cell membranes in IDDM. Relationship to glycemic control and microvascular complications. Diabetes 1998, 47, A127. [Google Scholar]
- Fu, M.X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The advanced glycation end product Ne-(Carboxymethyl)lysine is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem 1996, 271, 9982–9986. [Google Scholar]
- Anderson, M.M.; Requena, J.R.; Crowley, J.R.; Thorpe, S.R.; Heinecke, J.W. The myeloperoxidase system of human phagocytes generates Ne-(carboxymethyl)lysine on proteins: A mechanism for producing advanced glycation end products at sites of inflammation. J. Clin. Invest 1999, 104, 103–113. [Google Scholar]
- Prat, J.; Bellmunt, M.J.; Portero-Otín, M.; Pamplona, R. Fluorescent Products from Aminophospholipids and Glucose. In The Maillard Reaction in Foods and Medicine; O’brien, J., Nursten, H.E., Crabbe, M.J.C., Ames, J.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1998; p. 438. [Google Scholar]
- Al-Abed, Y.; Liebich, H.; Voelter, W.; Bucala, R. Hydroxyalkenal formation induced by advanced glycosylation of low density lipoprotein. J. Biol. Chem 1996, 271, 2892–2896. [Google Scholar]
- Obsil, T.; Amler, E.; Obsilova, V.; Pavlicek, Z. Effect of aminophospholipid glycation on order parameter and hydration of phospholipid bilayer. Biophys. Chem 1999, 80, 165–177. [Google Scholar]
- Ravandi, A.; Kuksis, A.; Shaikh, N.A. Glucosylated glycerophosphoethanolamines are the major LDL glycation products and increase LDL susceptibility to oxidation. Arterioscl. Throm. Vas. Biol 2000, 20, 467–477. [Google Scholar]
- Utzmann, C.M.; Lederer, M.O. Identification and quantification of aminophospholipid-linked Maillard compounds in model systems and egg yolk products. J. Agric. Food Chem 2000, 48, 1000–1008. [Google Scholar]
- Oak, J.-H.; Nakagawa, K.; Miyazawa, T. Synthetically prepared Amadori-glycated phosphatidylethanolamine can trigger lipid peroxidation via free radical reactions. FEBS Lett 2000, 481, 26–30. [Google Scholar]
- Breitling-Utzmann, C.M.; Unger, A.; Friedl, D.A.; Lederer, M.O. Identification and quantification of phosphatidylethanolamine-derived glucosylamines and aminoketoses from human erythrocytes-Influence of glycation products on lipid peroxidation. Archives Biochem. Biophys 2001, 391, 245–254. [Google Scholar]
- Lecompte, M-F.; Clavilier, J.; Rolland, C.; Collet, X.; Negre-Salvayre, A.; Salvayre, R. Effect of 4-hydroxynonenal on phosphatidylethanolamine containing condensed monolayer and on its interaction with apolipoprotein A-I. FEBS Lett. 2005, 579, 5074–5078. [Google Scholar]
- Levi, V.; Villamil Giraldo, A.M.; Castello, P.R.; Rossi, J.P.F.C.; Gonzalez Flecha, F.L. Effects of phosphatidylethanolamine glycation on lipid-protein interactions and membrane protein thermal stability. Biochem. J 2008, 416, 145–152. [Google Scholar]
- Simoes, C.; Simoes, V.; Reis, A.; Domingues, P.; Domingues, M.R.M. Oxidation of glycated phosphatidylethanolamines: Evidence of oxidation in glycated polar head identified by LC-MS/MS. Anal. Bioanal. Chem 2010, 397, 2417–2427. [Google Scholar]
- Simoes, C.; Domingues, P.; Domingues, M.R.M. Identification of free radicals in oxidized and glycoxidized phosphatidylethanolamines by spin trapping combined with tandem mass spectrometry. Rapid Commun. Mass Spectrom 2012, 26, 931–939. [Google Scholar]
- Herrmann, A.; Devaux, P.F. Alteration of the aminophospholipid translocase activity during in vivo and artificial aging of human erythrocytes. Biochim. Biophys. Acta 1990, 1027, 41–46. [Google Scholar]
- Zieseniss, S.; Zahler, S.; Müller, I.; Hermetter, A.; Engelmann, B. Modified phosphatidylethanolamine as the active component of oxidized low density lipoprotein promoting platelet prothrombinase activity. J. Biol. Chem 2001, 276, 19828–19835. [Google Scholar]
- Guichardant, M.; Bernoud-Hubac, N.; Chantegrel, B.; Deshayes, C.; Lagarde, M. Aldehydes from n-6 fatty acid peroxidation. Effects on aminophospholipids. Protag. Leukotr. Ess. Fatty Acids 2002, 67, 147–149. [Google Scholar]
- Oak, J.-H.; Nakagawa, K.; Oikawa, S.; Miyazawa, T. Amadori-glycated phosphatidylethanolamine induces angiogenic differentiations in cultured human umbilical vein endothelial cells. FEBS Lett 2003, 555, 419–423. [Google Scholar]
- Nakagawa, K.; Oak, J.-H.; Miyazawa, T. Angiogenic potency of Amadori-glycated phosphatidylethanolamine. Ann. N. Y. Acad. Sci 2005, 1043, 413–416. [Google Scholar]
- Bacot, S.; Bernoud-Hubac, N.; Chantegrel, B.; Deshayes, C.; Doutheau, A.; Ponsin, G.; Lagarde, M.; Guichardant, M. Evidence for in situ ethanolamine phospholipid adducts with hydroxyl-alkenals. J. Lipid Res 2007, 48, 816–825. [Google Scholar]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ 1998, 5, 551–562. [Google Scholar]
- Brenner, R.R. Effect of unsaturated acids on membrane structure and enzyme kinetics. Prog. Lipid Res 1984, 23, 69–96. [Google Scholar]
- Daum, G. Lipids of mitochondria. Biochim. Biophys. Acta 1985, 822, 1–42. [Google Scholar]
- Tyler, D. The Mitochondrion in Health and Disease; VCH Publishers: New York, NY, USA, 1992; pp. 78–80. [Google Scholar]
- Nakagawa, K.; Oak, J-H.; Higuchi, O.; Tsuzuki, T.; Oikawa, S.; Otani, H.; Mune, M.; Cai, H.; Miyazawa, T. Ion-trap tándem mass spectrometric analysis of Amadori-glycated phosphatidylethanolamine in human plasma with or without diabetes. J. Lipid Res. 2005, 46, 2514–2524. [Google Scholar]
- Miyazawa, T.; Oak, J.-H.; Nakagawa, K. tándem mass spectrometry analysis of Amadori-glycated phosphatidylethanolamine in human plasma. Ann. N. Y. Acad. Sci 2005, 1043, 280–283. [Google Scholar]
- Shoji, N.; Nakagawa, K.; Asai, A.; Fujita, I.; Hashiura, A.; Nakajima, Y.; Oikawa, S.; Miyazawa, T. LC-MS/MS analysis of carboxymethylated and carboxyethylated phosphatidylethanolamines in human erythrocytes and blood plasma. J. Lipid Res 2010, 51, 2445–2453. [Google Scholar]
- Sookwong, P.; Nakagawa, K.; Fujita, I.; Shoji, N.; Miyazawa, T. Amadori-glycated phosphatidylethanolamine, a potential marker for hyperglycemia, in streptozotocin-induced diabetic rats. Lipids 2011, 46, 943–952. [Google Scholar]
- Sell, D.R.; Lane, M.A.; Johnson, W.A.; Masoro, E.J.; Mock, O.B.; Reiser, K.M.; Fogarty, J.F.; Cutler, R.G.; Ingram, D.K.; Roth, G.S.; et al. Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. Proc. Natl. Acad. Sci. USA 1996, 93, 485–490. [Google Scholar]
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications. A new perspective on an old paradigm. Diabetes 1999, 48, 1–9. [Google Scholar]
- Prat, J.; Pamplona, R.; Sorribas, A.; Martín, S.; Viñallonga, M.; Segura, R. Correlation of plasma lipid fractions with colorimetrically determined glycated hemoglobin in a nondiabetic population. Metabolism 1989, 38, 1147–1153. [Google Scholar]
- Pamplona, R.; Belmunt, M.J.; Portero-Otín, M.; Prat, J. Mechanisms of glycation in atherogenesis. Med. Hypotheses 1993, 40, 174–181. [Google Scholar]
- Wautier, J.L.; Wautier, M.P.; Schmidt, A.M.; Anderson, G.M.; Hori, O.; Zoukourian, C.; Capron, L.; Chappey, O.; Yan, S.D.; Brett, J.; et al. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: A link between surface-associated AGEs and diabetic complications. Proc. Natl. Acad. Sci. USA 1994, 91, 7742–7746. [Google Scholar]
- Kislinger, T.; Fu, C.; Huber, B.; Qu, W.; Taguchi, A.; Du Yan, S.; Hofmann, M.; Yan, S.F.; Pischetsrieder, M.; Stern, D.; et al. N(epsilon)-(carboxymethyl)lysine adducts of proeins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Biol. Chem 1999, 274, 31740–31749. [Google Scholar]


| Experimental model | Analytical approach | Structural characterization | Ref. |
|---|---|---|---|
| In vitroStudies | |||
| Peroxidation of arachidonate and docosahexaenoate + synthetic dipalmitoyl phosphatidylethanolamine | Fluorimeter | Fluorescence chromophores (Ex 360 nm, Em 430 nm) | [31] |
| Rat liver mitochondrial and microsomal fractions peroxidized in vitro | Fluorimeter | Fluorescent chromophores (Ex 365–370 nm, Em 435–440 nm) | [47] |
| Methyl arachidonate/methyl linolenate/methyl linoleate/Malondialdehyde + phosphatidylethanolamine/phosphatidylserine (PE/PS) | Fluorimeter | Fluorescent chromophores (Ex 365 nm, Em 435 nm) | [32] |
| Malondialdehyde + Red blood Cells (RBC) | Fluorimeter | Fluorescent chromophores (Ex 390–400 nm, Em 460 nm) | [35] |
| Lipid peroxidation of rat liver microsomes | Fluorimeter | Fluorescent chromophores (Ex 359 nm, Em 430 nm) | [48] |
| Lipid peroxidation-derived compounds | Fluorimeter | Fluorescent chromophores (Ex 360 nm, Em 435 nm) | [33] |
| Malondialdehyde + primary amines | Fluorimeter | Identification of 1–4-Dihydropyridine-3,5-Dicarbaldehydes as model of fluorescent components in lipofuscin (Ex 375–405 nm, Em 435–465 nm) | [49] |
| 4-Hydroxynonenal (4-HNE) + microsomes/mictochondria/phospholipids (PS and PE) | Fluorimeter | Fluorescent chromophores (Ex 360 nm, Em 430 nm) | [45] |
| Malondialdehyde (MDA) + serine | Nuclear magnetic resonance (NMR) and (high performance liquid chromatography (HPLC) | N-2-(Propenal)serine | [50] |
| MDA + ethanolamine | NMR and HPLC | N-(2-propenal)ethanolamine | [51] |
| Mixed liposomes of l-alpha-dilinoleoyl-phosphatidylcholine (DiLinPC) and l-alpha-Dilinoleoylphosphatidylethanolamine (DiLinPE) in oxidative conditions | Fluorimeter and oxygen consumption monitored polarographically with a Clark-type oxygen probe | Fluorescent chromophores (Exc 360 nm, Em 430 nm) | [52] |
| Glucose + PE and PS | Liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS and TLC) | Glycated aminoglycerophospholipids | [53] |
| Lens + oxidative conditions | Fluorimeter and TLC | MDA:aminophospholipid adducts (Ex 360 nm, Em 470 nm) | [44] |
| Glycoxidation and autoxidation of PE from RBCs | Gas chromatography-mass spectrometry (GC-MS) | Carboxymethyl-ethanolamine (as marker of carboxymethyl-phosphatidylethanolamine, CM-PE) | [54] |
| Glucose + phosphatidylethanolamine | Gas-liquid chromatography-mass spectrometry (GLC-MS) and HPLC with diode-array detection (DAD) | 1-deoxy-1-(2-hydroxyethylamino)-D-fructose derivatives | [55] |
| Glucose + PE/phosphatidylcholine (PC)-PE liposomes | Thin layer chromatography (TLC), HPLC, NMR, fast atomic bombardment (FAB)-MS | Deoxy-D-fructosyl PE | [56] |
| (2-aminoethyl)phenetydylphosphate and (2-aminoethyl)ethylphosphate as model of aminophospholipids + carbohydrates | HPLC, NMR | Aminophospholipid glycation | [57] |
| PE and PS + glucose | GC-MS | N-(glucitol)ethanolamine and N-(carboxymethyl)serine | [58] |
| Human low density lipoprotein (hLDL) + p-hydroxyphenylacetaldehyde (p-hydroxyphenylacetaldehyde (pHA), product of l-tyrosine oxidation by the myeloperoxidase system of macrophages) | GC-MS | pHA-ethanolamine | [59] |
| Glucose and 3-Deoxyglucosone + PE | GLC-MS and HPLC-DAD | Formation of a phospholipid-linked pyrrolecarbaldehyde | [60] |
| Carbohydrates + PE | LC-ESI-MS, HPLC-DAD, and NMR | PE-derived Amadori compounds | [61] |
| PE + 4,5(E)-epoxy-2(E)-heptenal (secondary product of lipid peroxidation) | GC-MS, HPLC-MS, NMR | Phosphatidylethanolpyrroles and phosphatidylethanol-2-(1-hydroxypropyl)pyrroles | [62] |
| PE + 13-hydroperoxyoctadecadienoic acid and other oxidized poly unsaturated fatty acids (PUFAs) followed by phospholipase D-mediated hydrolysis | LC-MS and NMR | N-(hexanoyl)ethanolamine | [63] |
| Different species of PE + 4-hydroxy-trans-2-nonenal (4-HNE)/4-hydroxydodecadienal (4-HDDE)/4-hydroxyhexenal (4-HHE) | GC-MS, TLC, HPLC, and NMR | Aldehydes-PE. Different PE species are differently targeted by fatty aldehydes | [64] |
| PE + Isoketals (IsoK) | LC-ESI-MS | IsoK-PE pyrrole adducts and IsoK-PE Schiff base adducts | [65] |
| Fatty aldehydes released from plasmalogens after oxidation of cerebral cortex homogenates | GC-MS | N-heptadecyl-PE | [66] |
| PE + Glucose + Potential “antiglycative” compounds (protein glycation inhibitors, antioxidants, vitamins, etc.) | LC-ELSD-MS | Amadori-PE. Pyridoxal 5′-phosphate and pyridoxal/vitamin B6 derivatives) are the most effective antiglycative compounds | [67,68] |
| Acetoacetate + brain aminophospholipids | TLC and spectrophotometry | UV spectroscopy at 280 nm | [69] |
| Acetaldehyde-PE | Density functional theory study | Schiff base formation between PE and acetaldehyde | [70] |
| Liposomes and human high density lipoprotein (hHDL) particles in oxidative conditions | LC-MS | Isolevuglandins (IsoLGs)-, MDA-, 4.HNE-, N-Acyl-, and N-carboxyacyl-PEs | [71] |
| In vivoStudies | |||
| hLDL | Fluorimeter | Fluorescent chromophores AGEs-lipids (Ex 360 nm, Em 440 nm) | [72] |
| Rat liver aminophospholipids | GC-MS | Amadori aminophospholipids (as 5-(hydroxymethyl)-2-furfuraldehyde; 5-HMF) | [73] |
| Liver mitochondria from mammalian species | GC-MS | Carboxymethyl-ethanolamine (as marker of CM-PE) | [74] |
| hRBCs | TLC, HPLC, NMR, FAB-MS | Deoxy-D-fructosyl PE | [56] |
| RBC membranes | GC-MS | Glycation and carboxymethylation of aminophospholipids (PE and PS) | [75] |
| Glucose/lactose + PE; foods and biological samples Presence in foods (e.g., infant formula, chocolate) and in rat plasma | HPLC-UV (labeling with 3-methyl-2-benzothiazolinone hydrazone) | Glycated-PE and lactose-PE | [76] |
| RBC and LDL | LC-MS and NMR | N-(hexanoyl)ethanolamine | [63] |
| In vitro or in vivo experimental model | Analytical approach | Marker | Finding | Ref. |
|---|---|---|---|---|
| Rat and human urine | NMR and HPLC | N-2-(Propenal)serine | Direct evidence for oxidative decomposition of phospholipids by lipid peroxidation | [50] |
| Rat and human urine | NMR and HPLC | N-(2-propenal)ethanolamine | Direct evidence for oxidative decomposition of phospholipids by lipid peroxidation | [51] |
| Glucose + PE/hLDL | Fluorimeter | Fluorescent lipid advanced glycosylation (Ex 360 nm, Em 440 nm) | Increase of fluorescence associated with the progressive oxidative modification of unsaturated fatty acid residues | [72] |
| Lipids (PE and PS) and hLDL-advanced glycosylation | GC-MS | 4-hydroxyhexenal and 4-hydroxynonenal | Lipids-AGE formation in close proximity to unsaturated fatty acyl groups leads to lipid peroxidation | [85] |
| Unilamellar vesicles with PE and PC + glyceraldehyde | Time-resolved fluorescence spectroscopy | Aminophospholipid glycation increases the head-group hydration and lipid order in both regions of the membrane and lipid glycation is accompanied of lipid oxidation | [86] | |
| Atherosclerotic plaques collected from both diabetic and non-diabetic subjects | LC-ESI-MS | Glycated PE | Glycated aminophospholipids are the major LDL glycation products and increase LDL susceptibility to oxidation | [87] |
| Model systems and egg yolk products | LC-ESI-MS | Identification of PE-linked glucosylamines (Schiff-PE), Amadori products (Amadori-PE), 5-hydroxymethylpyrrole-2-carbaldehydes (Pyrrole-PE), and carboxymethyl- (CM-PE) as well as carboxyethyl-(CE-PE) derivatives | Possible influence on emulsifying properties and oxidation resistance | [88] |
| Amadori-PE + linoleic acid | LC-MS and colorimetry | TBARs and lipid hydroperoxides | Glycated-PE trigger lipid peroxidation via free radical reactions | [89] |
| RBCs from diabetic and healthy individuals | LC-ESI-MS | Schiff-PEs and Amadori-PEs, and detection of pyrrole-PE, CM-PE and CE-PE | Increase in diabetes; glycated PE promotes lipid peroxidation of biomembranes | [90] |
| Different species of PE + 4-HNE/4-HDDE/4-HHE | GC-MS, TLC, HPLC, and NMR | Aldehydes-PE | Different PE species are differently targeted by fatty aldehydes: the higher their hydrophobicity, the higher the amount of adducts made | [64] |
| PE/PC monolayers + 4-HNE | Alternating current (AC) polarography | Physico-chemical state of a condensed PE-containing phospholipid monolayer and its interaction with apo A-I | 4.HNE-PE does not alter monolayer stability, but decreases apo A-I insertion into the monolayer | [91] |
| PC/PE mixture + Glucose + isolated membrane proteins | Lipid-protein interactions | Amadori-PE and Amadori-proteins, and lipid-protein interaction parameters | Lipid glycation decreases the affinity of lipids for membrane proteins, induces structural rearrangements in the protein that makes it more sensitive to thermal unfolding and decreases the affinity between proteins and the surrounding phospholipids. | [92] |
| PE + Glucose + oxidative conditions | LC-ESI-MS | Glycated-PE + oxidation products | Oxidation of glycated-PE occurred more quickly than the oxidation of non-glycated-PE probably because of the existence of more oxidation sites derived from glycation of polar head group. | [93] |
| PE + Glucose + oxidative conditions | LC-MS-MS | Identification of free radicals in oxidized and glycoxidized PE | Presence of several sites susceptible to oxidation in glycated-PE which may be responsible for the increase in the oxidative reaction rate occurring in glycated compounds | [94] |
| Experimental model | Analytical approach | Marker | Finding | Reference |
|---|---|---|---|---|
| MDA+RBCs and in vitro lipid peroxidation of RBC | TLC | MDA:phospholipid adducts | Lipid peroxidation and MDA accumulation disturb organization of PS and PE in the human erythrocyte membrane bilayer | [38] |
| Erythrocytes of phenylhydrazine-treated rats | TLC | MDA:phospholipid adducts | Externalization of PS and PE in the membrane bilayer and hypercoagulability | [39] |
| Glucose-treated RBC | TLC | MDA:phospholipid adducts | Increase adduct formation and osmotic fragility in human RBCs | [42] |
| hRBCs from different age groups + MDA or H2O2 treatment | Aminophospholipid translocase activity | Decrease with age (defects in endogenous lipid asymmetry observed in aged human RBCs may be due to altered activity of the translocase) | [95] | |
| Lipid extracts from platelet incubated PE + PS + 4-HNE | LC-MS | PE-4-HNE compounds | Formation in cell membranes that could alter the phospholipase-dependent cell signalling | [46] |
| Glycated-PE LDL + THP1 cells (macrophages) | Cell culture, LC-ESI-MS | Glycated PE | Glycated-PtdEtn promotes macrophage uptake of LDL and accumulation of cholesteryl esters and triacylglycerols | [79] |
| Oxidized-LDL + platelets | TLC | Aldehyde-PE | Modified PE as the active component of oxidized LDL particles elicit a pronounced prothrombotic response by increasing the activity of the platelet prothrombinase complex | [96] |
| PE + 4-HNE and 4-HDDE (4-hydroxy-2E,6Z-dodecadienal) | TLC and GLC | 4-HNE-PE and 4-HDDE-PE | Modified PE is poor substrate for secreted phospholipase A2 | [97] |
| Human Plasma | TLC and GLC | 4-HNE-PE and 4-HDDE-PE | Potential alteration of phospholipid-dependent cell signaling | [97] |
| Amadori-PE + endothelial cells (HUVEC) | Cell culture | Cell proliferation, migration, and tube formation, and secretion of matrix metalloproteinase 2 (MMP-2) | Glycated-PE promotes vascular disease as a result of its angiogenic activity on endothelial cells | [98,99] |
| Human blood platelets | GC-MS | 4-HHE-, 4-HNE-, and 4-HDDE-PE | In vivo identification. Increase of platelet aggregation | [100] |
| Experimental model | Analytical approach | Marker | Finding | Reference |
|---|---|---|---|---|
| Aged RBC | Fluorimeter | Fluorescent chromophores (Ex 390–400 nm, Em 460 nm) | Increase with aging Altered physical and biochemical properties of aging cells (polymerization of plasma membrane proteins) | [35] |
| Lipids extracts from different tissues (heart, brain, liver, testis, kidney, adrenal cortex) | Fluorimeter | Fluorescent chromophores | Increase with aging, and in pathological conditions (e.g., diabetes, hyperlipidemia) | [15,34] |
| RBC fromiron-deficient infants and animals | Thin Layer Chromatography (TLC) | Schiff’s base adduct due to reaction MDA + PS/PE | Decrease RBC survival | [36] |
| RBC from the “sickle cell disease” | Fluorimeter and TLC | Fluoresecent chromophores & MDA:aminophospholipid adducts | Increase adduct formation in sickle cell disease | [37] |
| Lipid extracts of the human cataractous and normal lenses | Fluorimeter and TLC | Fluoresecnt chromophores (Ex 365 nm, Em 460 nm) and MDA: aminophospholipid adducts | Increase in human senile cataract | [43] |
| Aged human RBC membranes | Fluorimeter and TLC | Fluorescent chromophores & MDA: aminophospholipid adducts | Increase with aging | [40] |
| RBC from diabetic patients | TLC | MDA:phospholipid adducts | Increase adducts in diabetes | [41] |
| humanRBC (hRBC) from different age groups + MDA or H2O2 treatment | Aminophospholipid translocase activity | Decrease with age (defects in endogenous lipid asymmetry observed in aged human RBC may be due to altered activity of the translocase) | [96] | |
| LDL from diabetic patients | Fluorimeter | Increase in diabetes | [72] | |
| Rat liver aminophospholipids in streptozotocin-induce diabetic rats | GC-MS | Amadori aminophospholipids (as 5-(hydroxymethyl)-2- furfuraldehyde; 5-HMF) | Increase in diabetes | [73] |
| RBC and plasma from diabetic and control subjects | LC-ESI-MS | Glycated aminophospholipids | Increase in diabetes | [77] |
| Urine from diabetic and control subjects | GC-MS | Carboxymethylethanolamine (as marker of CM-PE) | No increase in diabetes | [54] |
| Liver mitochondria from mammalian species | GC-MS | Carboxymethyl-ethanolamine (as marker of CM-PE) | CM-PE formation at mitochondrial level is associated with animal lifespan | [74] |
| hRBC from diabetic and control subjects | GC-MS | N-(glucitol)ethanolamine & N-(carboxymethyl)serine | Adducts formed in vivo and increased in diabetes | [58] |
| hLDL from plasma and atherosclerotic aorta | GC-MS | pHA-ethanolamine | Increase of pHA-PE in human atherosclerotic intima | [59] |
| RBC from diabetic and healthy individuals | LC-ESI-MS | Schiff-PEs, Amadori-PEs, pyrrole-PE, CM-PE and CE-PE | Increase in diabetes; glycated PE promotes lipid peroxidation of biomembranes | [90] |
| Amadori-PE of a lipid extract from diabetic plasma | QTRAP LC-MS-MS | Amadori-PE | Increase in diabetes | [105] |
| Plasma from: healthy volunteers, chronic hemodyalisis patients, Type II diabetic patients without chronic hemodialysis, and Type II diabetic patients with chronic hemodialysis | HPLC-MS-MS | Amadori-PE | Increase of Amadori-PE in diabetes with or without chronic hemodialysis | [106] |
| Plasma from streptozotocin-diabetic rats | LC-ELSD-MS | Amadori-PE | Increase in diabetes and decrease in streptozotocin-induced diabetes and pyridoxal-treated rats | [67,68] |
| Retinas of streptozotocin-induced diabetic rats | GC-MS | 4-HHE-, 4-HNE-, and 4-HDDE-PE | Increase in diabetic animals | [101] |
| hRBC and blood plasma from healthy subjects and diabetic patients | LC-MS-MS | CM-PE and CE-PE as AGE-PE, and Amadori-PE | No significant differences were observed in AGE-PE in RBC and plasma, whereas Amadori-PE concentrations were higher in diabetic patients | [107] |
| Blood plasma, kidney, RBCs, liver, pancreas, cerebrum, and cerebellum from streptozotocin-induced diabetes rats | LC-MS-MS | Amadori-PE | Increase in diabetes. Amadori-PE(18:0–20:4) is the PE species that acts as the most sensitive indicator | [108] |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Naudí, A.; Jové, M.; Ayala, V.; Cabré, R.; Portero-Otín, M.; Pamplona, R. Non-Enzymatic Modification of Aminophospholipids by Carbonyl-Amine Reactions. Int. J. Mol. Sci. 2013, 14, 3285-3313. https://doi.org/10.3390/ijms14023285
Naudí A, Jové M, Ayala V, Cabré R, Portero-Otín M, Pamplona R. Non-Enzymatic Modification of Aminophospholipids by Carbonyl-Amine Reactions. International Journal of Molecular Sciences. 2013; 14(2):3285-3313. https://doi.org/10.3390/ijms14023285
Chicago/Turabian StyleNaudí, Alba, Mariona Jové, Victòria Ayala, Rosanna Cabré, Manuel Portero-Otín, and Reinald Pamplona. 2013. "Non-Enzymatic Modification of Aminophospholipids by Carbonyl-Amine Reactions" International Journal of Molecular Sciences 14, no. 2: 3285-3313. https://doi.org/10.3390/ijms14023285





