Potential of Nitrogen Gas (N2) Flushing to Extend the Shelf Life of Cold Stored Pasteurised Milk
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microbiological Analyses
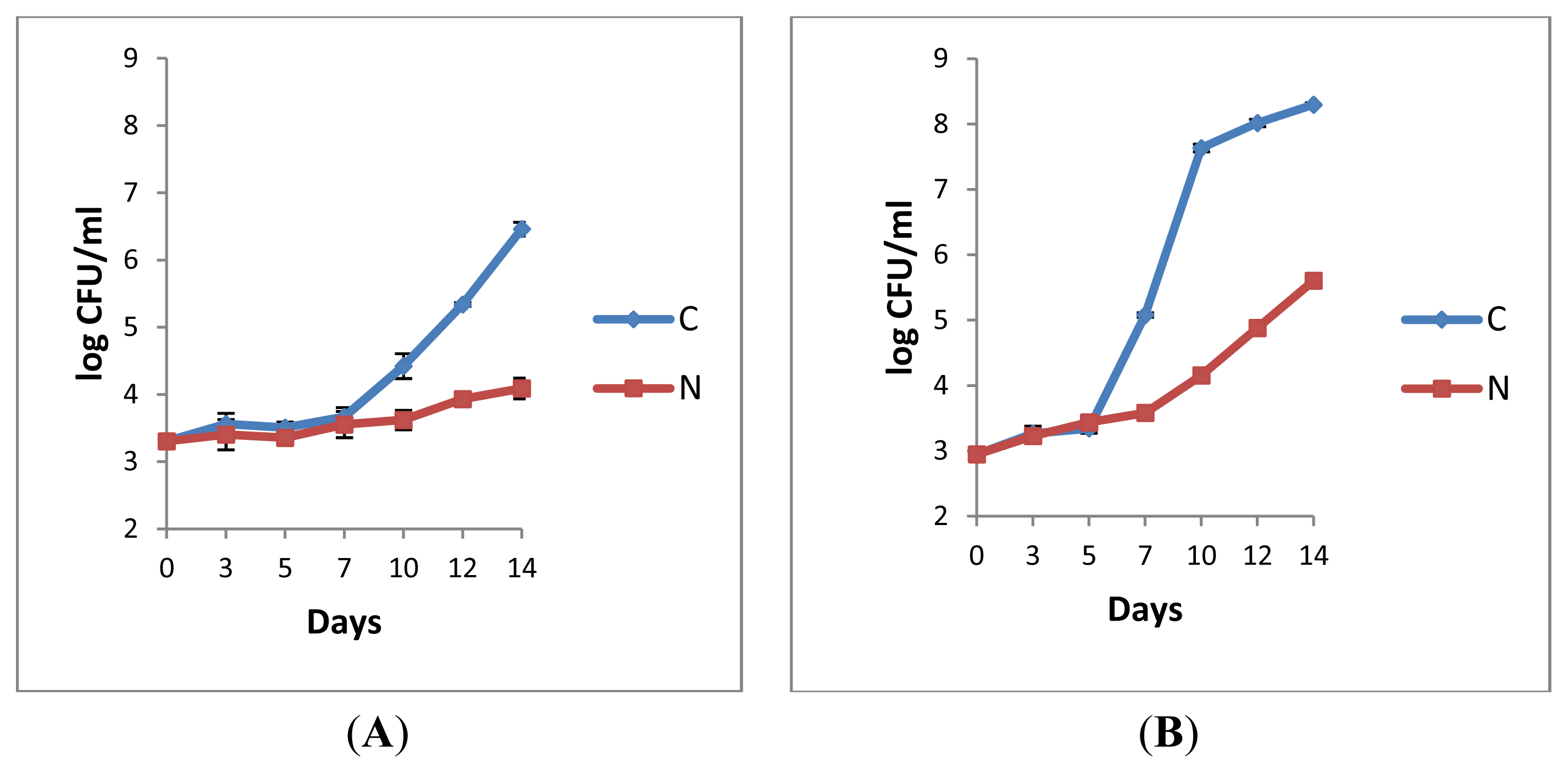
2.1.1. Retail Pasteurised Milk
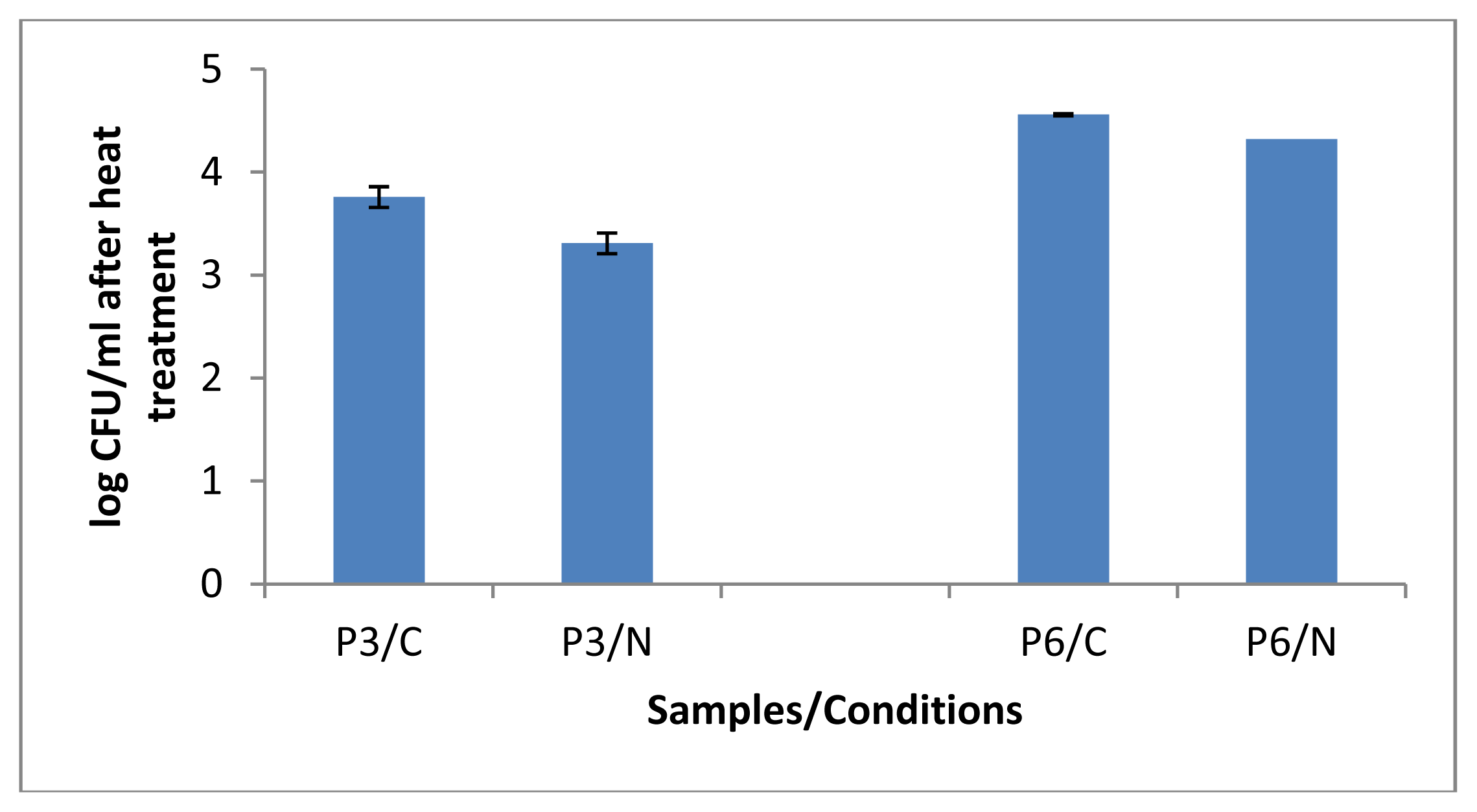
2.1.2. Bacterial Counts Following Heat Treatments of Milk Samples
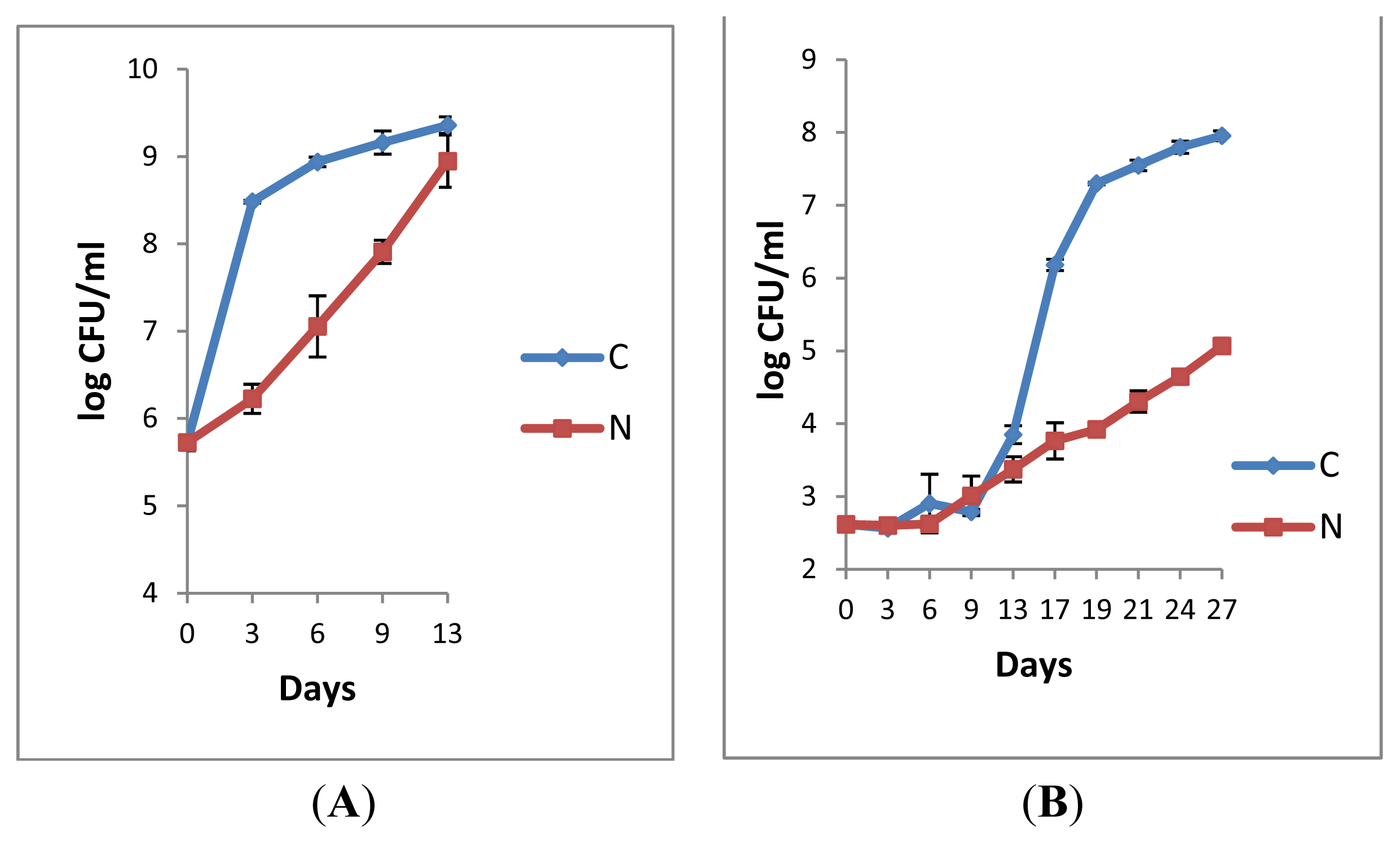
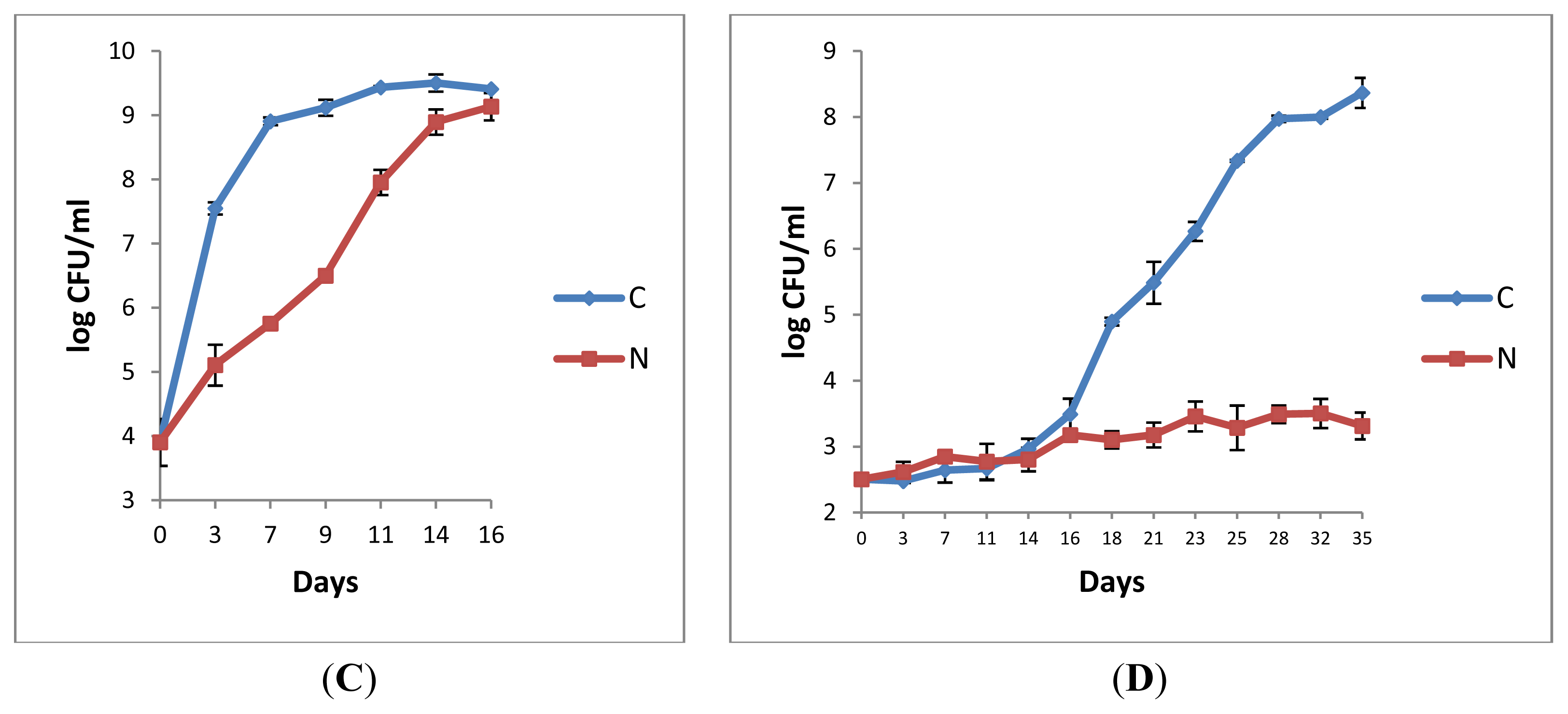
2.1.3. Microbiological Analyses of Raw Milk and the Corresponding Pasteurised Milk
2.2. Characterisation of Selected Bacterial Isolates by 16 rRNA and rpoB Gene Sequences
3. Experimental Section
3.1. Procurement of Raw and Pasteurised Milk Samples
3.2. N2 Gas Treatments
3.3. Microbiological Analyses of Milk
3.4. Evaluation of Aerobic Spore Levels at the End of the N2-Flushing
3.5. DNA-Based Characterisation of Bacterial Isolates
3.5.1. Selection of Isolates
3.5.2. DNA Extraction
3.5.3. Partial Sequencing of the 16S rRNA and rpoB Genes
4. Conclusions
Acknowledgments
References
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sporng, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F. Food-borne diseases. The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol 2010, 139, S3–S15. [Google Scholar]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: the challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar]
- Tudge, C. Enlightened agriculture. Food Ethics Council 2012, 7, 15–16. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste. Presented at Save Food Congress. Interpack 2011 and FAO, Düsseldorf, Germany, May 2011.
- DEFRA. 2012. Available online: http://www.defra.gov.uk accessed on 15 August 2012.
- Chambers, J.V. The Microbiologyof Raw Milk. In Dairy Microbiology Handbook, 3rd ed.; Robinson, R.K., Ed.; Wiley-Interscience: New York, NY, USA, 2002; pp. 39–89. [Google Scholar]
- Munsch-Alatossava, P.; Alatossava, T. Antibiotic resistance of raw-milk associated psychrotrophic bacteria. Microbiol. Res 2007, 162, 115–123. [Google Scholar]
- Munsch-Alatossava, P.; Gauchi, J.P.; Chamlagain, B.; Alatossava, T. Trends of antibiotic resistance in mesophilic and psychrotrophic bacterial populations during cold storage of raw milk. ISRN Microbiol. 2012, 2012. [Google Scholar] [CrossRef]
- Munsch-Alatossava, P.; Ikonen, V.; Alatossava, T.; Gauchi, J.P. Trends of Antibiotic Resistance (AR) in Mesophilic and Psychrotrophic Bacterial Populations During Cold Storage of Raw Milk, Produced by Organic and Conventional Farming Systems. In Antibiotic Resistant Bacteria, A Continuous Challenge in the New Millenium; Pana, M., Ed.; Intech: Rijeka, Croatia, 2012; pp. 105–124. [Google Scholar]
- Meunier-Goddik, L.; Sandra, S. Pasteurised Milk Products. In Encyclopedia of Dairy Sciences; Roginski, H., Fuquay, J.W., Fox, P.F., Eds.; Academic Press: New York, NY, USA, 2002; Volume 3, pp. 1627–1637. [Google Scholar]
- Olivier, S.P.; Jayarao, B.M.; Almeida, R.A. Foodborne pathogens in milk and the dairy farm environment: Food safety and public health implications. Foodborne Pathog. Dis 2005, 2, 115–129. [Google Scholar]
- U.S. Food and Drug Administration. Grade “A” Pasteurised milk ordinance (PMO). 2005. Available online: http://fda.gov/food/foodsafety/Product-SpecificInformation/MilkSafety/ accessed on 15 August 2012.
- Hillerton, J.E.; Berry, E.A. Quality of the milk supply: European regulations versus practice. NMC Annual meeting Proceedings. 2004. Available online: http://nmconline.org/articles/qualityeuro.pdf accessed on 15 August 2012.
- Ranieri, M.L.; Huck, J.R.; Sonnen, M.; Barbano, D.M.; Boor, K.J. High temperature, short time pasteurization temperatures inversely affect bacterial numbers during refrigerated storage of pasteurised fluid milk. J. Dairy Sci 2012, 92, 4823–4832. [Google Scholar]
- Petrus, R.R.; Loiola, C.G.; Oliveira, C.A.F. Microbiological shelf life of pasteurised milk in bottle and pouch. J. Food Sci 2009, 75, M36–M40. [Google Scholar]
- Ranieri, M.L.; Boor, K.J. Short communication: Bacterial ecology of high temperature, short time pasteurized milk processed in the United States. J. Dairy Sci 2009, 92, 4833–4840. [Google Scholar]
- Ivy, R.A.; Ranieri, M.L.; Martin, N.H.; Den Bakker, H.C.; Xavier, B.M.; Wiedmann, M.; Boor, K.J. Identification and characterization of psychrotolerant sporeformers associated with fluid milk production and processing. Appl. Environ. Microbiol 2012, 78, 1853–1864. [Google Scholar]
- Huck, J.R.; Sonne, M.; Boor, K.J. Tracking heat-resistant, cold thriving fluid milk spoilage bacteria from farm to packaged product. J. Dairy Sci 2009, 91, 1218–1228. [Google Scholar]
- Silliker, J.H.; Wolfe, S.K. Microbiological safety considerations in controlled-atmosphere storage of meats. Food Technol 1980, 34, 59–63. [Google Scholar]
- King, J.S.; Mabbitt, L.A. Preservation of raw milk by the addition of carbon dioxide. J. Dairy Res 1982, 49, 439–447. [Google Scholar]
- Hotchkiss, J.H.; Lee, E. Extending the shelf life of dairy products with dissolved carbon dioxide. Eur. Dairy Mag 1996, 8, 1–19. [Google Scholar]
- Ruas-Madieodo, P.; Bada-Gancedo, J.C.; Fernandez-Garcia, E.; Gonzalez de Lano, D.; de Los Reyes-Gavilan, C.G. Preservation of the microbiological and biochemical quality of raw milk by carbon dioxide addition: A pilot- scale study. J. Food Prot 1996, 59, 502–508. [Google Scholar]
- Rajagopal, M.; Werner, B.G.; Hotchkiss, J.H. Low pressure CO2 storage of raw milk: Microbiological effects. J. Dairy Sci 2005, 88, 3130–3138. [Google Scholar]
- Dechemi, S.; Benjelloun, H.; Lebeault, J.M. Effect of modified atmospheres on the growth and extracellular enzymes of psychrotrophs in raw milk. Eng. Life Sci 2005, 5, 350–356. [Google Scholar]
- Murray, S.K.; Kwan, K.K.H.; Skura, B.J.; Mc Kellar, R.C. Effect of nitrogen flushing on the production of proteinase by psychrotrophic bacteria in raw milk. J. Food Sci 1983, 48, 1166–1169. [Google Scholar]
- Munsch-Alatossava, P.; Gursoy, O.; Alatossava, T. Exclusion of phospholipases (PLs)-producing bacteria in raw milk flushed with nitrogen gas (N2). Microbiol. Res 2010, 165, 61–65. [Google Scholar]
- Munsch-Alatossava, P.; Gursoy, O.; Alatossava, T. Potential of nitrogen gas (N2) to control psychrotrophs and mesophiles in raw milk. Microbiol. Res 2010, 165, 122–132. [Google Scholar]
- Munsch-Alatossava, P.; Gursoy, O.; Alatossava, T. Improved storage of cold raw milk by continuous flushing of N2 gas separated from compressed air: A pilot scale study. J. Food Process. Technol. 2011, 1, 101. [Google Scholar] [CrossRef]
- Munsch-Alatossava, P.; Alatossava, T. Controlled Atmosphere-based Improved Storage of Cold Raw Milk: Potential of N2 Gas. In Food Storage; Cohen, G.E., Levin, C.M., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 99–116. [Google Scholar]
- Munsch-Alatossava, P.; Ghafar, A.; Gursoy, O.; Alatossava, T. Changes in bacterial populations present in raw and pasteurised milks under N2 gas flushing. to be submitted for publication.
- Berge, O.; Guinebretière, M.H.; Achouak, W.; Normand, P.; Heulin, T. Paenibacillus graminis sp. nov. and Paenibacillus odorifer sp. nov, isolated from plant roots, soil and food. Int. J Syst. Evol. Microbiol 2002, 52, 607–616. [Google Scholar]
- Da Mota, F.F.; Gomes, E.A.; Paiva, E.; Rosado, A.S.; Seldin, L. Use of rpoB gene analysis for identification of nitrogen-fixing Paenibacillus species as an alternative to the 16S rRNA gene. Lett. Appl. Microbiol 2004, 39, 34–40. [Google Scholar]
- Lechner, S.; Mayr, R.; Francis, K.P.; Prüβ, B.M.; Kaplan, T.; Wieβner-Gunkel, E.; Stewart, G.S.A.B.; Scherer, S. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int. J. Syst. Bacteriol 1998, 48, 1373–1382. [Google Scholar]
- Réjasse, A.; Gilois, N.; Barbosa, I.; Huillet, E.; Bevilacqua, C.; Tran, S.; Ramarao, N.; Stenfors Arnesen, L.P.; Sanchis, V. Temperature-dependent production of various PlcR-controlled virulence factors in Bacillus weihenstephanensis strain KBAB4. Appl. Environ. Microbiol 2012, 78, 2553–2561. [Google Scholar]
- Schmiel, D.H.; Miller, V.L. Bacterial phospholipases and pathogenesis. Microbes Infect 1999, 1, 1103–1112. [Google Scholar]
- Dogan, B.; Boor, K.J. Genetic diversity and spoilage potentials among Pseudomonas spp. isolated from fluid milk products and dairy processing plants. Appl. Environ. Microbiol 2003, 69, 130–138. [Google Scholar]
- Munsch-Alatossava, P.; Alatossava, T. Phenotypic characterization of raw-milk associated psychrotrophic bacteria. Microbiol. Res 2006, 161, 334–346. [Google Scholar]
- Ogier, J.C.; Son, O.; Gruss, A.; Tailliez, P.; Delacroix-Buchet, A. Identification of the bacterial microflora in dairy products by temporal temperature gradient gel electrophoresis. Appl. Environ. Microbiol 2002, 68, 3691–3701. [Google Scholar]
- Dahllöf, I.; Baillie, H.; Kjelleberg, S. rpoB-based microbial community analysis avoids limitations inherent in 16S rRNA gene intraspecies heterogeneity. Appl. Environ. Microbiol 2000, 66, 3376–3380. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol 1990, 215, 403–410. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. Mega 5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol 2011, 28, 2731–2739. [Google Scholar]
| Isolates 1 | Spoilage Features 2 | Analyses of Gene Sequences | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 16S rRNA | rpoB | ||||||||
| PR | LP | PL | Species | Acc. number in GenBank | % ID 3 | Species | Acc. number in GenBank | % ID 3 | |
| P3Nd3 | − | − | − | Microbacterium lacticum | NR_026160.1 | 99 | ND | ||
| Microbacterium schleiferi | NR_044936.1 | 98 | |||||||
| Microbacterium flavum | NR_041562.1 | ||||||||
| P3Na7 | + | − | + | Bacillus weihenstephanensis | NR_024697.1 | 99 | Bacillus weihenstephanensis | CP000903.1 | 98 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P3Ca14 | + | − | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 98 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P3Cb14 | + | − | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 99 |
| B. mycoides | NR_036880.1 | 99 | |||||||
| B. thuringiensis | NR_043403.1 | 98 | |||||||
| B. anthracis | NR_041248.1 | 99 | |||||||
| P3Na14 | + | − | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 98 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P3Nb14 | + | − | + | B. weihenstephanensis | NR_024697.1 | 99 | B.weihenstephanensis | CP000903.1 | 99 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P3Nc14 | + | − | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 98 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P3Nd14 | + | − | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 99 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P6Na14 | + | + | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 98 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P6Nb14 | + | + | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 99 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P6Nc14 | + | + | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 99 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P6Nd14 | + | + | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 98 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P6Ne14 | + | + | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 99 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P6Nf14 | + | + | + | B. weihenstephanensis | NR_024697.1 | 99 | ND | ||
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P6Ng14 | + | + | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 99 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| P6Nh14 | + | + | + | B. weihenstephanensis | NR_024697.1 | 100 | ND | ||
| B. mycoides | NR_036880.1 | 99 | |||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| L2PNa | + | + | − | B. sonorensis | NR_025130.1 | 98 | ND | ||
| B. aerius | NR_042338.1 | ||||||||
| L2PNb | + | + | + | B. weihenstephanensis | NR_024697.1 | 99 | B. cereus | EF607283.1 | 99 |
| B. mycoides | NR_036880.1 | B. thuringiensis | CP001903.1 | 100 | |||||
| B. thuringiensis | NR_043403.1 | B. cereus | FJ188319.1 | 99 | |||||
| B. anthracis | NR_041248.1 | B. cereus | AE016877.1 | 100 | |||||
| L2PNa6 | + | + | − | B. sonorensis | NR_025130.1 | 98 | B. licheniformis | CP000002.3 | 96 |
| B. subtilis ssp. subtilis | NR_027552.1 | B. licheniformis | AE017333.1 | 96 | |||||
| L2PNa9 | + | + | − | B. aerophilus | NR_042339.1 | 99 | ND | ||
| B. pumilus | NR_043242.1 | ||||||||
| L2PNa13 | ɛ | + | − | B. sonorensis | NR_025130.1 | 98 | ND | ||
| B. subtilis ssp. subtilis | NR_027552.1 | 97 | |||||||
| L2PNa17 | + | + | + | B. weihenstephanensis | NR_024697.1 | 99 | B. weihenstephanensis | CP000903.1 | 99 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| L2PCa21 | − | + | − | Paenibacillus odorifer | NR_028887.1 | 99 | Paenibacillus odorifer | AY493862.1 | 99 |
| L2PNa27 | + | + | + | B. weihenstephanensis | NR_0246971 | 99 | B. weihenstephanensis | CP000903.1 | 99 |
| B. mycoides | NR_036880.1 | ||||||||
| B. thuringiensis | NR_043403.1 | ||||||||
| B. anthracis | NR_041248.1 | ||||||||
| Milk samples | Origin |
|---|---|
| P3 | Retail pasteurised milk (Arla Ingman Ltd, Finland) |
| P6 | Retail pasteurised milk (Arla Ingman Ltd, Finland) |
| L2R | Raw milk |
| L2P | Raw milk L2R pasteurised (73 °C/15 s) at the pilot plant / Univ. of Helsinki |
| L3R | Raw milk |
| L3P | Raw milk L3R pasteurised (73 °C/15 s) at the pilot plant / Univ. of Helsinki |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Munsch-Alatossava, P.; Ghafar, A.; Alatossava, T. Potential of Nitrogen Gas (N2) Flushing to Extend the Shelf Life of Cold Stored Pasteurised Milk. Int. J. Mol. Sci. 2013, 14, 5668-5685. https://doi.org/10.3390/ijms14035668
Munsch-Alatossava P, Ghafar A, Alatossava T. Potential of Nitrogen Gas (N2) Flushing to Extend the Shelf Life of Cold Stored Pasteurised Milk. International Journal of Molecular Sciences. 2013; 14(3):5668-5685. https://doi.org/10.3390/ijms14035668
Chicago/Turabian StyleMunsch-Alatossava, Patricia, Abdul Ghafar, and Tapani Alatossava. 2013. "Potential of Nitrogen Gas (N2) Flushing to Extend the Shelf Life of Cold Stored Pasteurised Milk" International Journal of Molecular Sciences 14, no. 3: 5668-5685. https://doi.org/10.3390/ijms14035668




