Protein Tyrosine Nitration and Thiol Oxidation by Peroxynitrite—Strategies to Prevent These Oxidative Modifications
Abstract
:1. Introduction
2. Results
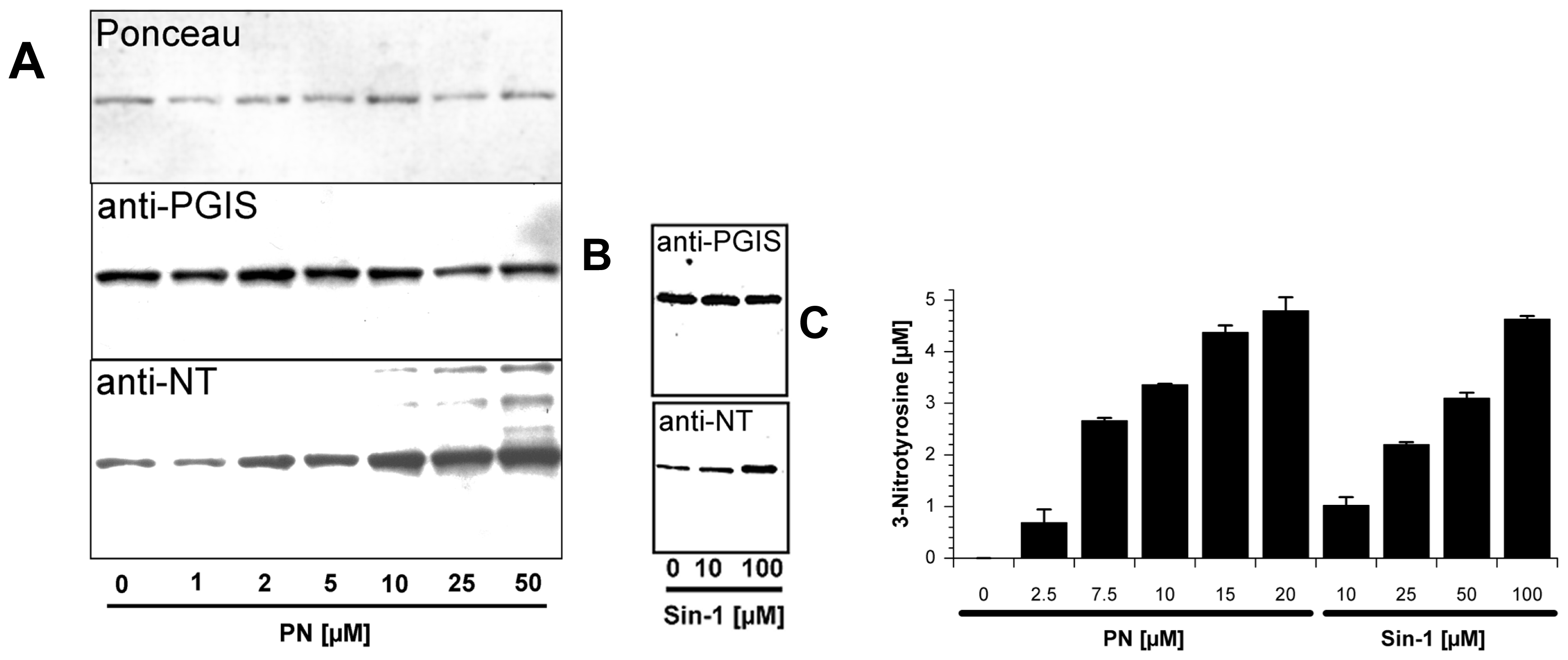
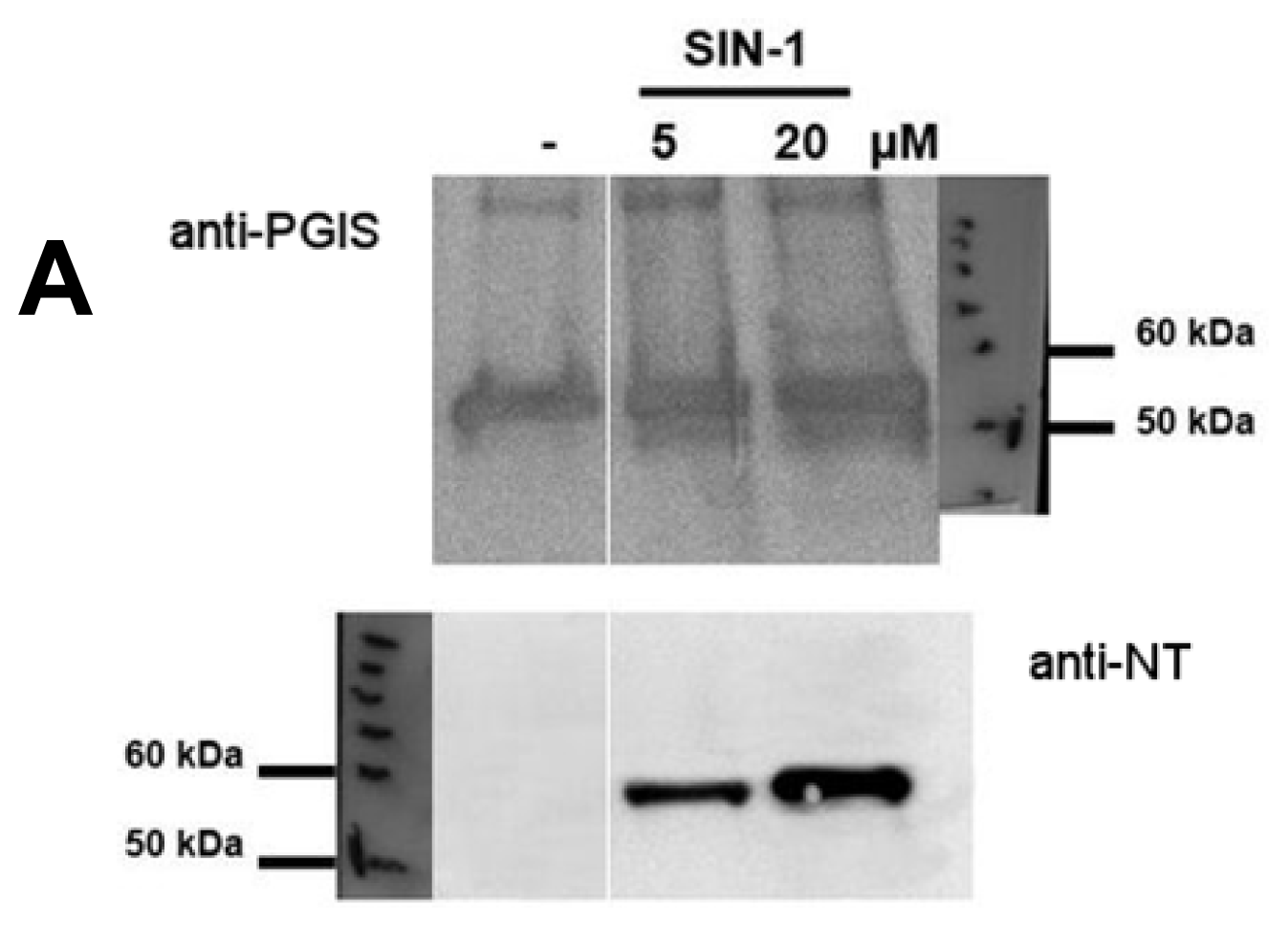
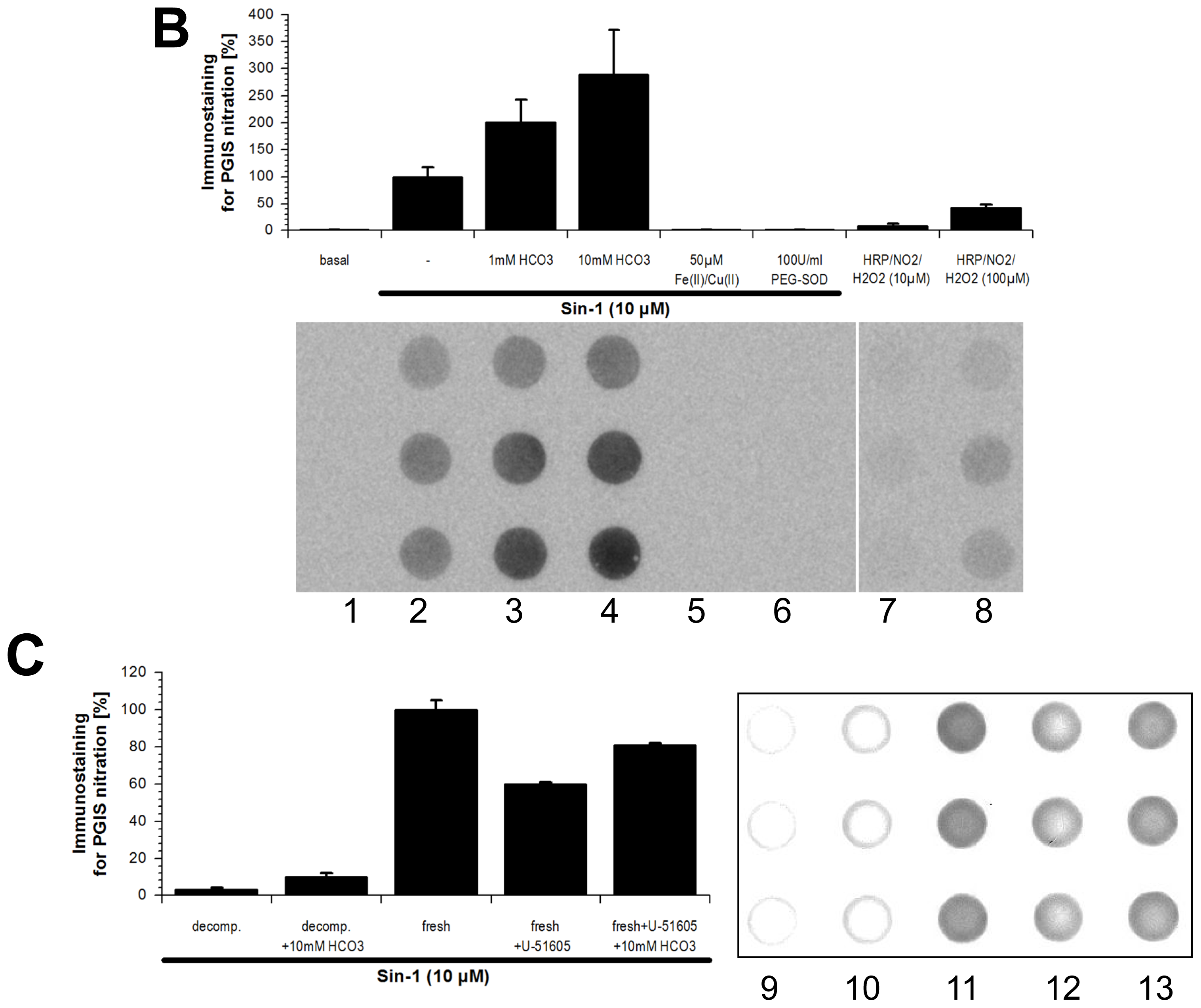
2.1. Tyrosine Nitration of PGIS
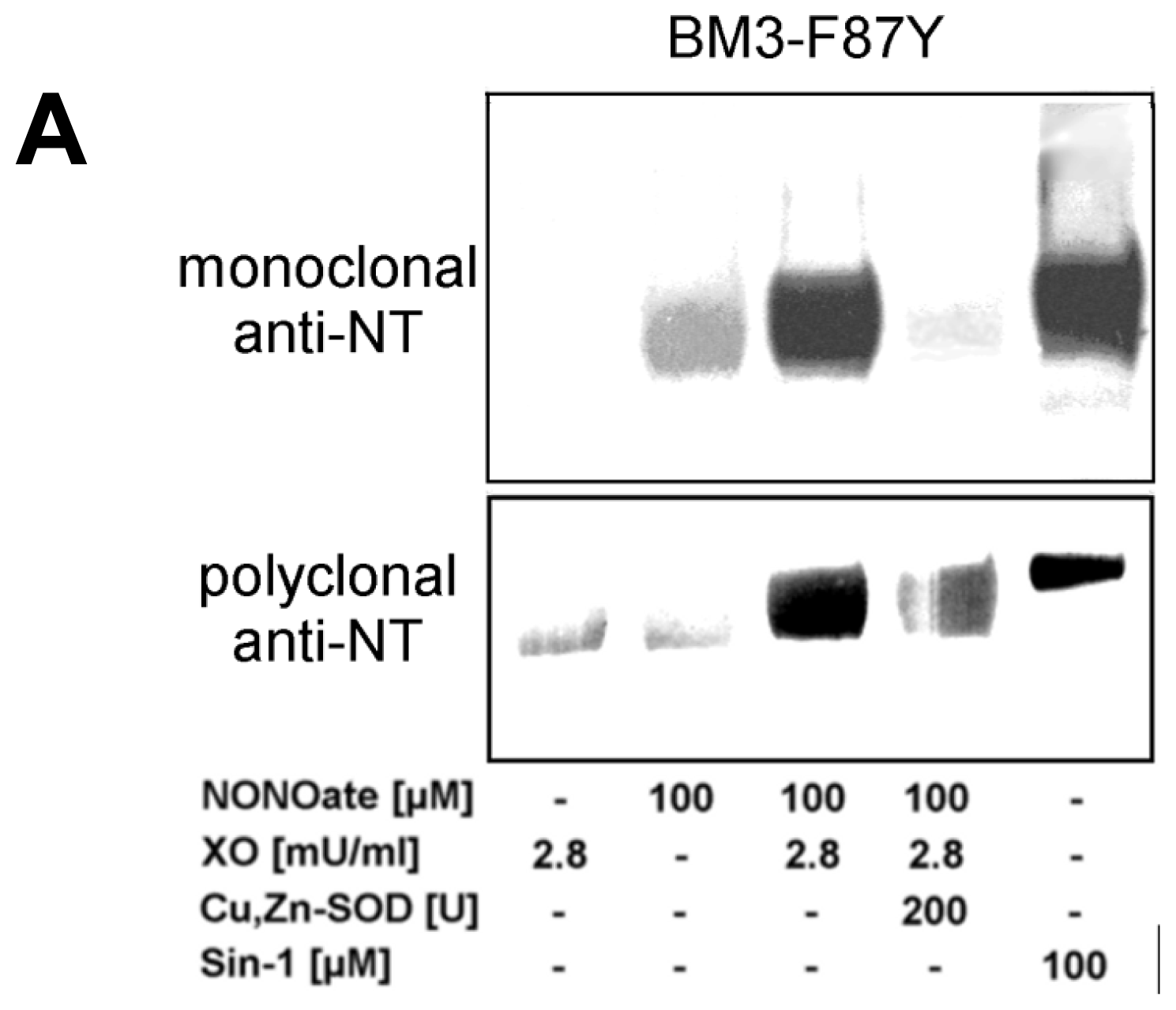
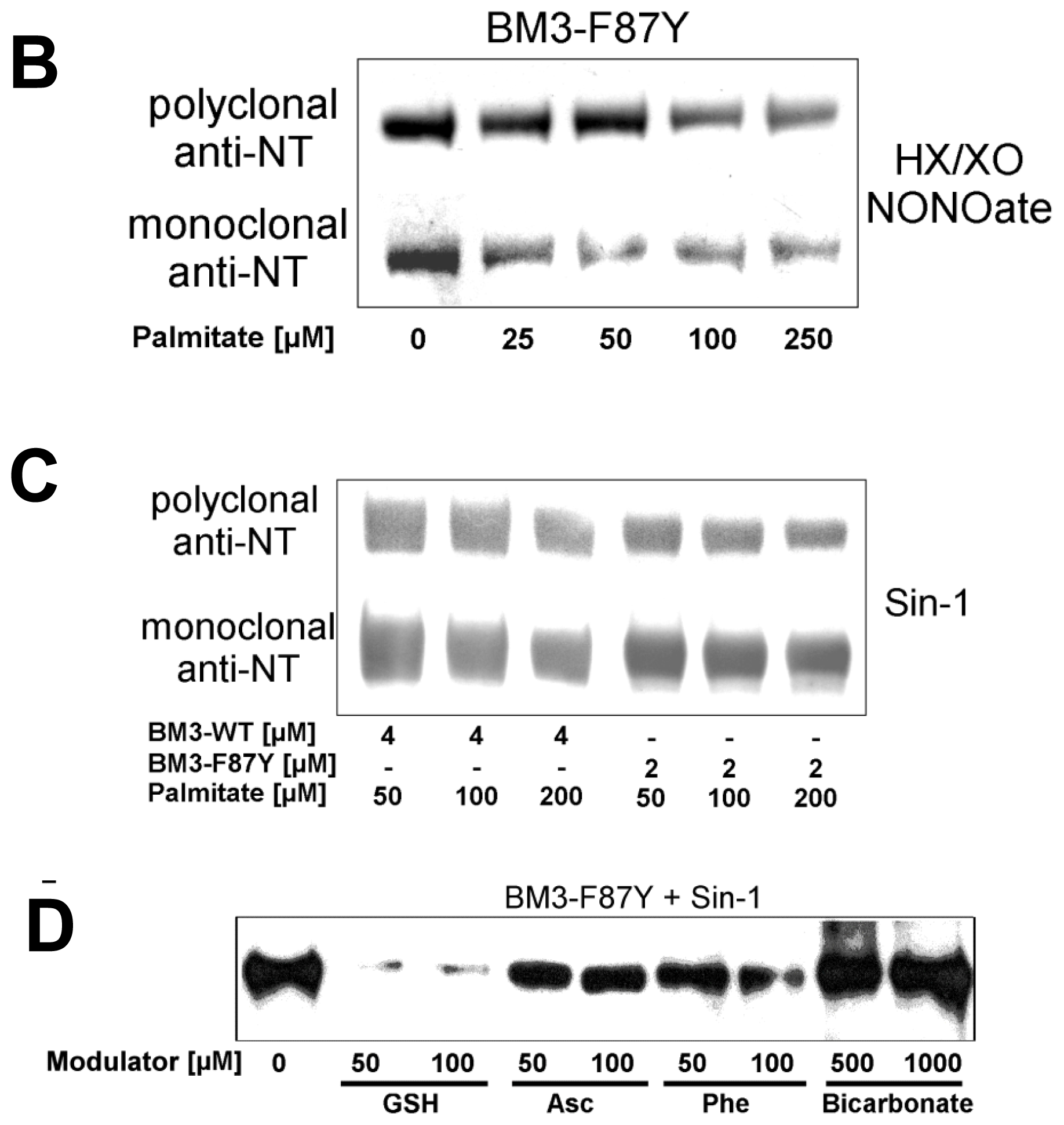
2.2. Nitration of P450BM-3 by in Situ Generated Peroxynitrite from Sin-1 or Xanthine Oxidase/Spermine NONOate
2.3. Scavengers of Peroxynitrite Inhibiting the Nitration and Hydroxylation of Phenol
2.4. Scavengers of Peroxynitrite Inhibiting the Nitrosation of Phenol
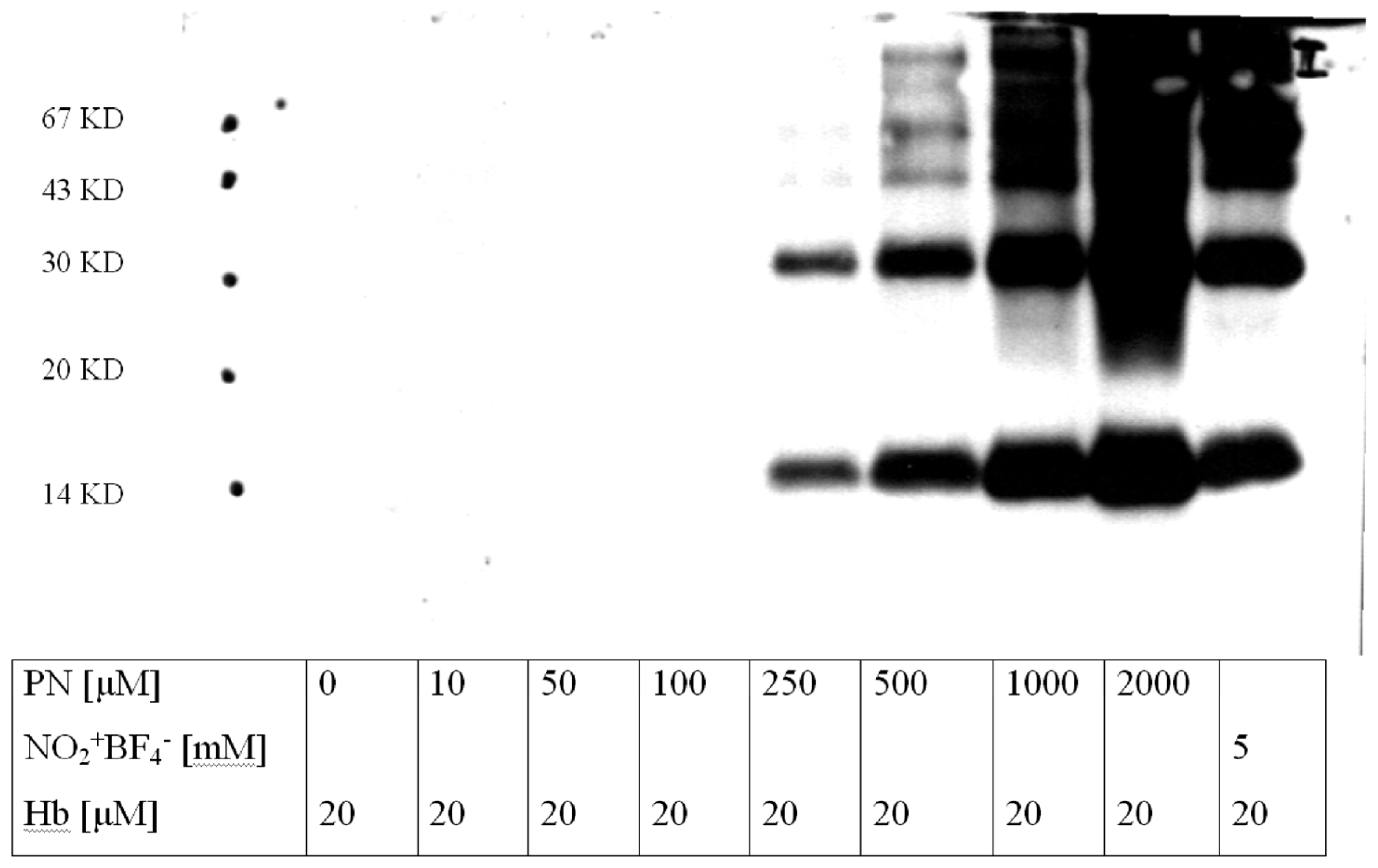
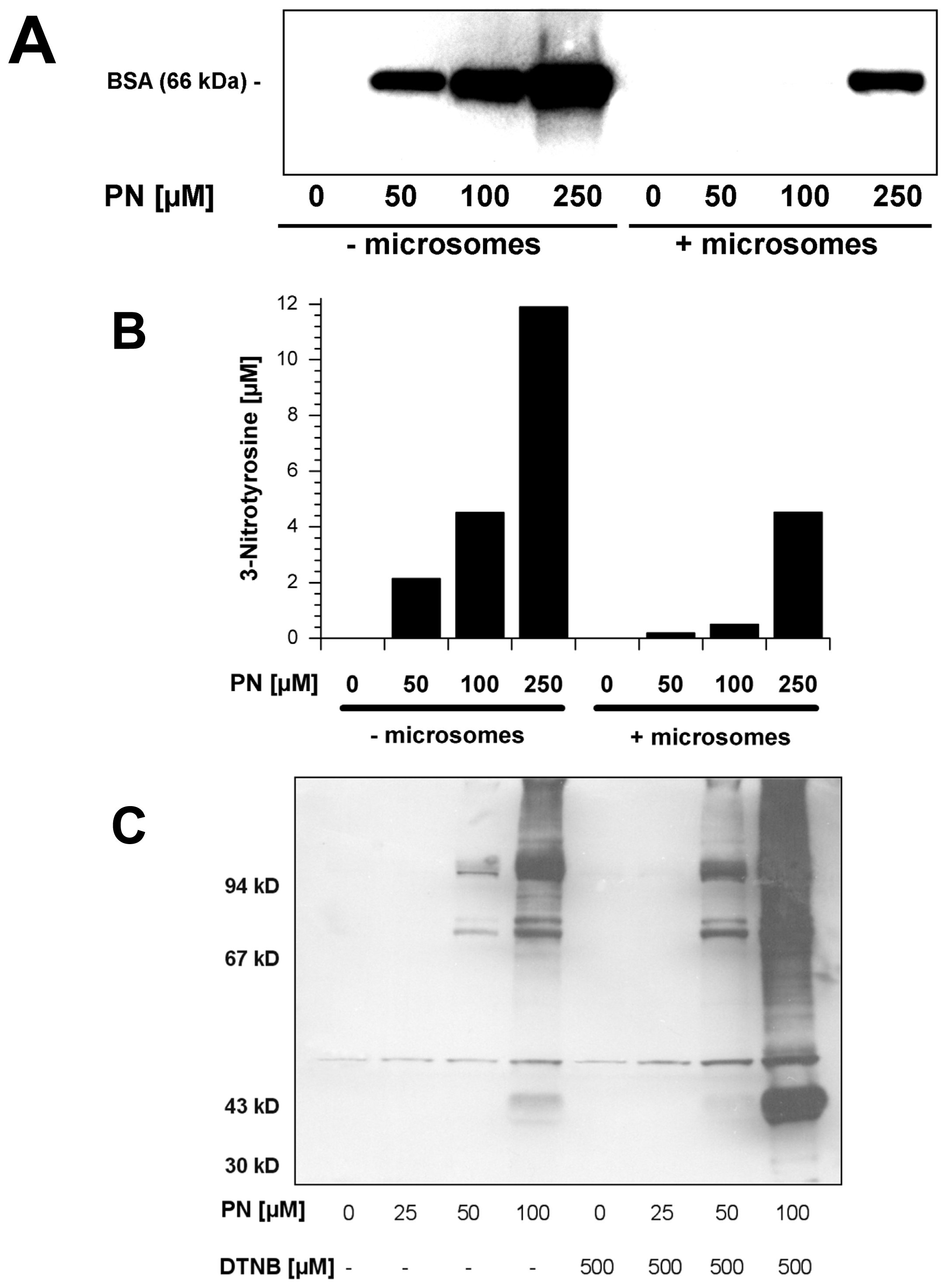
2.5. Scavengers of Peroxynitrite Inhibiting the Nitration of BSA
2.6. Scavengers of Peroxynitrite Inhibiting the Metal-Catalyzed Nitration of Phenol
2.7. Scavengers of Peroxynitrite Inhibiting the Oxidation and Inactivation of Alcohol Dehydrogenase
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Nitration of Purified, Recombinant PGIS Protein by in Situ Generated Peroxynitrite from Sin-1
4.3. Nitration of P450BM-3 Proteins and PGIS by either Authentic Peroxynitrite or in Situ Generated Peroxynitrite from Sin-1 or Xanthine Oxidase/Spermine NONOate
4.4. Inhibition of Nitration by Peroxynitrite Scavengers and Substrate Analogue Inhibitors
4.5. Western Blot Analysis of Nitrated Proteins
4.6. Proteolytic Digestion of Nitrated Proteins by Pronase and Direct Detection of 3-Nitrotyrosine
4.7. Stability of the Nitrated Peptide from PGIS and Specificity of Cleavage by Thermolysine
4.8. Alcohol Dehydrogenase Activity
4.9. Nitration of Phenol
4.10. Metal-Catalyzed Nitration of Phenol
4.11. Kinetics of Peroxynitrite-Decomposition
5. Conclusions
Supplementary Information
ijms-14-07542-s001.pdfAcknowledgements
Abbreviations
| PGIS | prostacyclin synthase |
| MnSOD | manganese superoxide dismutase |
| NOS | nitric oxide synthase |
| P450BM-3 | cytochrome P450 bacterial monooxygenase-3 |
| P450CAM | cytochrome P450 camphor-5-monooxygenase |
| Sin-1 | 3-morpholino sydnonimine |
| BSA | bovine serum albumin |
| ADH | alcohol dehydrogenase |
| MP-11 | microperoxidase |
Conflict of Interest
References and Notes
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar]
- Daiber, A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta 2010, 1797, 897–906. [Google Scholar]
- Munzel, T.; Daiber, A.; Ullrich, V.; Mulsch, A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cgmp-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol 2005, 25, 1551–1557. [Google Scholar]
- Crow, J.P.; Beckman, J.S. Reaction between nitric oxide, superoxide, and peroxynitrite: Footprints of peroxynitrite in vivo. Adv. Pharmacol 1995, 35, 17–43. [Google Scholar]
- Ferdinandy, P. Peroxynitrite: Just an oxidative/nitrosative stressor or a physiological regulator as well? Br. J. Pharmacol 2006, 148, 1–3. [Google Scholar]
- Tien, M.; Berlett, B.S.; Levine, R.L.; Chock, P.B.; Stadtman, E.R. Peroxynitrite-mediated modification of proteins at physiological carbon dioxide concentration: pH dependence of carbonyl formation, tyrosine nitration, and methionine oxidation. Proc. Natl. Acad. Sci. USA 1999, 96, 7809–7814. [Google Scholar]
- Viner, R.I.; Williams, T.D.; Schoneich, C. Peroxynitrite modification of protein thiols: Oxidation, nitrosylation, and s-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum ca-atpase. Biochemistry 1999, 38, 12408–12415. [Google Scholar]
- Daiber, A.; Bachschmid, M. Enzyme inhibition by peroxynitrite-mediated tyrosine nitration and thiol oxidation. Curr. Enzyme Inhib 2007, 3, 103–117. [Google Scholar]
- Turko, I.V.; Murad, F. Protein nitration in cardiovascular diseases. Pharmacol. Rev 2002, 54, 619–634. [Google Scholar]
- Smith, M.A.; Richey Harris, P.L.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread peroxynitrite-mediated damage in alzheimer’s disease. J. Neurosci 1997, 17, 2653–2657. [Google Scholar]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev 2007, 87, 315–424. [Google Scholar]
- Ferdinandy, P.; Schulz, R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol 2003, 138, 532–543. [Google Scholar]
- Ullrich, V.; Kissner, R. Redox signaling: Bioinorganic chemistry at its best. J. Inorg. Biochem 2006, 100, 2079–2086. [Google Scholar]
- Ischiropoulos, H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem. Biophys. Res. Commun 2003, 305, 776–783. [Google Scholar]
- Greenacre, S.A.; Ischiropoulos, H. Tyrosine nitration: Localisation, quantification, consequences for protein function and signal transduction. Free Radic. Res 2001, 34, 541–581. [Google Scholar]
- Brennan, M.L.; Wu, W.; Fu, X.; Shen, Z.; Song, W.; Frost, H.; Vadseth, C.; Narine, L.; Lenkiewicz, E.; Borchers, M.T.; et al. A tale of two controversies: Defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem 2002, 277, 17415–17427. [Google Scholar]
- Ischiropoulos, H.; Beckman, J.S. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J. Clin. Invest 2003, 111, 163–169. [Google Scholar]
- Kooy, N.W.; Royall, J.A.; Ye, Y.Z.; Kelly, D.R.; Beckman, J.S. Evidence for in vivo peroxynitrite production in human acute lung injury. Am. J. Respir. Crit. Care Med 1995, 151, 1250–1254. [Google Scholar]
- Ischiropoulos, H. Biological tyrosine nitration: A pathophysiological function of nitric oxide and reactive oxygen species. Arch. Biochem. Biophys 1998, 356, 1–11. [Google Scholar]
- Beckman, J.S. Protein tyrosine nitration and peroxynitrite. FASEB J 2002, 16, 1144. [Google Scholar]
- Pinzar, E.; Wang, T.; Garrido, M.R.; Xu, W.; Levy, P.; Bottari, S.P. Angiotensin ii induces tyrosine nitration and activation of erk1/2 in vascular smooth muscle cells. FEBS Lett 2005, 579, 5100–5104. [Google Scholar]
- Csibi, A.; Communi, D.; Muller, N.; Bottari, S.P. Angiotensin ii inhibits insulin-stimulated glut4 translocation and akt activation through tyrosine nitration-dependent mechanisms. PLoS One 2010, 5, e10070. [Google Scholar]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol 1996, 271, C1424–C1437. [Google Scholar]
- Daiber, A.; Ullrich, V. Peroxynitrite reactions with heme and heme-thiolate (p450) proteins. Methods Enzymol 2002, 359, 379–389. [Google Scholar]
- Daiber, A.; Bachschmid, M.; Beckman, J.S.; Munzel, T.; Ullrich, V. The impact of metal catalysis on protein tyrosine nitration by peroxynitrite. Biochem. Biophys. Res. Commun 2004, 317, 873–881. [Google Scholar]
- Van der Vliet, A.; Eiserich, J.P.; O’Neill, C.A.; Halliwell, B.; Cross, C.E. Tyrosine modification by reactive nitrogen species: A closer look. Arch. Biochem. Biophys 1995, 319, 341–349. [Google Scholar]
- Floris, R.; Piersma, S.R.; Yang, G.; Jones, P.; Wever, R. Interaction of myeloperoxidase with peroxynitrite. A comparison with lactoperoxidase, horseradish peroxidase and catalase. Eur. J. Biochem 1993, 215, 767–775. [Google Scholar]
- Zou, M.; Martin, C.; Ullrich, V. Tyrosine nitration as a mechanism of selective inactivation of prostacyclin synthase by peroxynitrite. Biol. Chem 1997, 378, 707–713. [Google Scholar]
- Zou, M.; Yesilkaya, A.; Ullrich, V. Peroxynitrite inactivates prostacyclin synthase by heme-thiolate-catalyzed tyrosine nitration. Drug Metab. Rev 1999, 31, 343–349. [Google Scholar]
- Gwozdz, P.; Drelicharz, L.; Kozlovski, V.I.; Chlopicki, S. Prostacyclin, but not nitric oxide, is the major mediator of acetylcholine-induced vasodilatation in the isolated mouse heart. Pharmacol. Rep 2007, 59, 545–552. [Google Scholar]
- Mehl, M.; Daiber, A.; Herold, S.; Shoun, H.; Ullrich, V. Peroxynitrite reaction with heme proteins. Nitric Oxide 1999, 3, 142–152. [Google Scholar]
- Daiber, A.; Herold, S.; Schoneich, C.; Namgaladze, D.; Peterson, J.A.; Ullrich, V. Nitration and inactivation of cytochrome p450bm-3 by peroxynitrite. Stopped-flow measurements prove ferryl intermediates. Eur. J. Biochem 2000, 267, 6729–6739. [Google Scholar]
- Daiber, A.; Schoneich, C.; Schmidt, P.; Jung, C.; Ullrich, V. Autocatalytic nitration of p450cam by peroxynitrite. J. Inorg. Biochem 2000, 81, 213–220. [Google Scholar]
- Meli, R.; Nauser, T.; Latal, P.; Koppenol, W.H. Reaction of peroxynitrite with carbon dioxide: Intermediates and determination of the yield of co3*- and no2*. J. Biol. Inorg. Chem 2002, 7, 31–36. [Google Scholar]
- Goldstein, S.; Czapski, G.; Lind, J.; Merenyi, G. Carbonate radical ion is the only observable intermediate in the reaction of peroxynitrite with co(2). Chem. Res. Toxicol 2001, 14, 1273–1276. [Google Scholar]
- Bonini, M.G.; Radi, R.; Ferrer-Sueta, G.; Ferreira, A.M.; Augusto, O. Direct epr detection of the carbonate radical anion produced from peroxynitrite and carbon dioxide. J. Biol. Chem 1999, 274, 10802–10806. [Google Scholar]
- Souza, J.M.; Daikhin, E.; Yudkoff, M.; Raman, C.S.; Ischiropoulos, H. Factors determining the selectivity of protein tyrosine nitration. Arch. Biochem. Biophys 1999, 371, 169–178. [Google Scholar]
- Zou, M.H.; Daiber, A.; Peterson, J.A.; Shoun, H.; Ullrich, V. Rapid reactions of peroxynitrite with heme-thiolate proteins as the basis for protection of prostacyclin synthase from inactivation by nitration. Arch. Biochem. Biophys 2000, 376, 149–155. [Google Scholar]
- Boccini, F.; Herold, S. Mechanistic studies of the oxidation of oxyhemoglobin by peroxynitrite. Biochemistry 2004, 43, 16393–16404. [Google Scholar]
- Schmidt, P.; Youhnovski, N.; Daiber, A.; Balan, A.; Arsic, M.; Bachschmid, M.; Przybylski, M.; Ullrich, V. Specific nitration at tyrosine 430 revealed by high resolution mass spectrometry as basis for redox regulation of bovine prostacyclin synthase. J. Biol. Chem 2003, 278, 12813–12819. [Google Scholar]
- Zou, M.; Jendral, M.; Ullrich, V. Prostaglandin endoperoxide-dependent vasospasm in bovine coronary arteries after nitration of prostacyclin synthase. Br. J. Pharmacol 1999, 126, 1283–1292. [Google Scholar]
- MacMillan-Crow, L.A.; Crow, J.P.; Kerby, J.D.; Beckman, J.S.; Thompson, J.A. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl. Acad. Sci. USA 1996, 93, 11853–11858. [Google Scholar]
- MacMillan-Crow, L.A.; Crow, J.P.; Thompson, J.A. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 1998, 37, 1613–1622. [Google Scholar]
- Cassina, A.M.; Hodara, R.; Souza, J.M.; Thomson, L.; Castro, L.; Ischiropoulos, H.; Freeman, B.A.; Radi, R. Cytochrome c nitration by peroxynitrite. J. Biol. Chem 2000, 275, 21409–21415. [Google Scholar]
- Radi, R.; Cassina, A.; Hodara, R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol. Chem 2002, 383, 401–409. [Google Scholar]
- Takakura, K.; Beckman, J.S.; MacMillan-Crow, L.A.; Crow, J.P. Rapid and irreversible inactivation of protein tyrosine phosphatases ptp1b, cd45, and lar by peroxynitrite. Arch. Biochem. Biophys 1999, 369, 197–207. [Google Scholar]
- Daiber, A.; Frein, D.; Namgaladze, D.; Ullrich, V. Oxidation and nitrosation in the nitrogen monoxide/superoxide system. J. Biol. Chem 2002, 277, 11882–11888. [Google Scholar]
- Pryor, W.A.; Jin, X.; Squadrito, G.L. One- and two-electron oxidations of methionine by peroxynitrite. Proc. Natl. Acad. Sci. USA 1994, 91, 11173–11177. [Google Scholar]
- Perrin, D.; Koppenol, W.H. The quantitative oxidation of methionine to methionine sulfoxide by peroxynitrite. Arch. Biochem. Biophys 2000, 377, 266–272. [Google Scholar]
- Pfeiffer, S.; Schmidt, K.; Mayer, B. Dityrosine formation outcompetes tyrosine nitration at low steady-state concentrations of peroxynitrite. Implications for tyrosine modification by nitric oxide/superoxide in vivo. J. Biol. Chem 2000, 275, 6346–6352. [Google Scholar]
- Goldstein, S.; Czapski, G.; Lind, J.; Merenyi, G. Tyrosine nitration by simultaneous generation of (•)no and O-(2) under physiological conditions. How the radicals do the job. J. Biol. Chem 2000, 275, 3031–3036. [Google Scholar]
- Pfeiffer, S.; Lass, A.; Schmidt, K.; Mayer, B. Protein tyrosine nitration in cytokine-activated murine macrophages. Involvement of a peroxidase/nitrite pathway rather than peroxynitrite. J. Biol. Chem 2001, 276, 34051–34058. [Google Scholar]
- Schildknecht, S.; Heinz, K.; Daiber, A.; Hamacher, J.; Kavakli, C.; Ullrich, V.; Bachschmid, M. Autocatalytic tyrosine nitration of prostaglandin endoperoxide synthase-2 in lps-stimulated raw 264.7 macrophages. Biochem. Biophys. Res. Commun 2006, 340, 318–325. [Google Scholar]
- Bachschmid, M.; Schildknecht, S.; Ullrich, V. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem. Biophys. Res. Commun 2005, 338, 536–542. [Google Scholar]
- Whiteman, M.; Ketsawatsakul, U.; Halliwell, B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann. N. Y. Acad. Sci 2002, 962, 242–259. [Google Scholar]
- Robinson, K.M.; Morre, J.T.; Beckman, J.S. Triuret: A novel product of peroxynitrite-mediated oxidation of urate. Arch. Biochem. Biophys 2004, 423, 213–217. [Google Scholar]
- Masumoto, H.; Sies, H. The reaction of ebselen with peroxynitrite. Chem. Res. Toxicol 1996, 9, 262–267. [Google Scholar]
- Daiber, A.; Zou, M.H.; Bachschmid, M.; Ullrich, V. Ebselen as a peroxynitrite scavenger in vitro and ex vivo. Biochem. Pharmacol 2000, 59, 153–160. [Google Scholar]
- 3-morpholinosydnonimine (Sin-1) is the active metabolite of Molsidomin, a nitrovasodilator and cardiovascular drug, which upon hydrolysis undergoes autoxidation by molecular oxygen and thereby generates superoxide anion radicals. The resulting Sin-1 cation radical undergoes fragmentation and yields nitric oxide and Sin-1C. Since the formation of superoxide and nitric oxide is stoichiometric, these radicals almost quantitatively form peroxynitrite in a diffusion controlled reaction. Since the release of peroxynitrite from Sin-1 under physiological conditions is slow, this compound is frequently used to mimic biological fluxes of nitric oxide, superoxide and peroxynitrite.
- Ferrer-Sueta, G.; Batinic-Haberle, I.; Spasojevic, I.; Fridovich, I.; Radi, R. Catalytic scavenging of peroxynitrite by isomeric mn (iii) n-methylpyridylporphyrins in the presence of reductants. Chem. Res. Toxicol 1999, 12, 442–449. [Google Scholar]
- Crow, J.P. Manganese and iron porphyrins catalyze peroxynitrite decomposition and simultaneously increase nitration and oxidant yield: Implications for their use as peroxynitrite scavengers in vivo. Arch. Biochem. Biophys 1999, 371, 41–52. [Google Scholar]
- Daiber, A.; Mehl, M.; Ullrich, V. New aspects in the reaction mechanism of phenol with peroxynitrite: The role of phenoxy radicals. Nitric Oxide 1998, 2, 259–269. [Google Scholar]
- Lymar, S.V.; Khairutdinov, R.F.; Hurst, J.K. Hydroxyl radical formation by O–O bond homolysis in peroxynitrous acid. Inorg. Chem 2003, 42, 5259–5266. [Google Scholar]
- Van der Vliet, A.; O’Neill, C.A.; Halliwell, B.; Cross, C.E.; Kaur, H. Aromatic hydroxylation and nitration of phenylalanine and tyrosine by peroxynitrite. Evidence for hydroxyl radical production from peroxynitrite. FEBS Lett 1994, 339, 89–92. [Google Scholar]
- Ferdinandy, P.; Schulz, R. Inhibition of peroxynitrite-induced dityrosine formation with oxidized and reduced thiols, nitric oxide donors, and purine derivatives. Antioxid. Redox Signal 2001, 3, 165–171. [Google Scholar]
- Carroll, R.T.; Galatsis, P.; Borosky, S.; Kopec, K.K.; Kumar, V.; Althaus, J.S.; Hall, E.D. 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (tempol) inhibits peroxynitrite-mediated phenol nitration. Chem. Res. Toxicol 2000, 13, 294–300. [Google Scholar]
- Crow, J.P.; Beckman, J.S.; McCord, J.M. Sensitivity of the essential zinc-thiolate moiety of yeast alcohol dehydrogenase to hypochlorite and peroxynitrite. Biochemistry 1995, 34, 3544–3552. [Google Scholar]
- Kissner, R.; Nauser, T.; Bugnon, P.; Lye, P.G.; Koppenol, W.H. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem. Res. Toxicol 1997, 10, 1285–1292. [Google Scholar]
- Epe, B.; Ballmaier, D.; Roussyn, I.; Briviba, K.; Sies, H. DNA damage by peroxynitrite characterized with DNA repair enzymes. Nucleic Acids Res 1996, 24, 4105–4110. [Google Scholar]
- Darley-Usmar, V.M.; Hogg, N.; O’Leary, V.J.; Wilson, M.T.; Moncada, S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic. Res. Commun 1992, 17, 9–20. [Google Scholar]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys 1991, 288, 481–487. [Google Scholar]
- Rubbo, H.; Radi, R.; Trujillo, M.; Telleri, R.; Kalyanaraman, B.; Barnes, S.; Kirk, M.; Freeman, B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem 1994, 269, 26066–26075. [Google Scholar]
- Moore, K.P.; Darley-Usmar, V.; Morrow, J.; Roberts, L.J., 2nd. Formation of f2-isoprostanes during oxidation of human low-density lipoprotein and plasma by peroxynitrite. Circ. Res. 1995, 77, 335–341. [Google Scholar]
- Khor, H.K.; Fisher, M.T.; Schoneich, C. Potential role of methionine sulfoxide in the inactivation of the chaperone groel by hypochlorous acid (HOCl) and peroxynitrite (ONOO-). J. Biol. Chem 2004, 279, 19486–19493. [Google Scholar]
- Sharov, V.S.; Schoneich, C. Diastereoselective protein methionine oxidation by reactive oxygen species and diastereoselective repair by methionine sulfoxide reductase. Free Radic. Biol. Med 2000, 29, 986–994. [Google Scholar]
- Beckmann, J.S.; Ye, Y.Z.; Anderson, P.G.; Chen, J.; Accavitti, M.A.; Tarpey, M.M.; White, C.R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe Seyler 1994, 375, 81–88. [Google Scholar]
- Wayenberg, J.L.; Ransy, V.; Vermeylen, D.; Damis, E.; Bottari, S.P. Nitrated plasma albumin as a marker of nitrative stress and neonatal encephalopathy in perinatal asphyxia. Free Radic. Biol. Med 2009, 47, 975–982. [Google Scholar]
- Wayenberg, J.L.; Cavedon, C.; Ghaddhab, C.; Lefevre, N.; Bottari, S.P. Early transient hypoglycemia is associated with increased albumin nitration in the preterm infant. Neonatology 2011, 100, 387–397. [Google Scholar]
- Zou, M.H.; Ullrich, V. Peroxynitrite formed by simultaneous generation of nitric oxide and superoxide selectively inhibits bovine aortic prostacyclin synthase. FEBS Lett 1996, 382, 101–104. [Google Scholar]
- Kozak, A.J.; Liu, F.; Funovics, P.; Jacoby, A.; Kubant, R.; Malinski, T. Role of peroxynitrite in the process of vascular tone regulation by nitric oxide and prostanoids—A nanotechnological approach. Prostaglandins Leukot. Essent. Fatty Acids 2005, 72, 105–113. [Google Scholar]
- Zou, M.H.; Klein, T.; Pasquet, J.P.; Ullrich, V. Interleukin 1beta decreases prostacyclin synthase activity in rat mesangial cells via endogenous peroxynitrite formation. Biochem. J 1998, 336, 507–512. [Google Scholar]
- Spisni, E.; Griffoni, C.; Santi, S.; Riccio, M.; Marulli, R.; Bartolini, G.; Toni, M.; Ullrich, V.; Tomasi, V. Colocalization prostacyclin (pgi2) synthase—Caveolin-1 in endothelial cells and new roles for pgi2 in angiogenesis. Exp. Cell Res 2001, 266, 31–43. [Google Scholar]
- Bachschmid, M.; Ullrich, V. Redox signalling in endothelial cells—Novel mechanisms explaining endothelium function and dysfunction in health, disease and aging. B.I.F. FUTURA 2003, 18, 223–230. [Google Scholar]
- Schildknecht, S.; Bachschmid, M.; Ullrich, V. Peroxynitrite provides the peroxide tone for pghs-2-dependent prostacyclin synthesis in vascular smooth muscle cells. FASEB J 2005, 19, 1169–1171. [Google Scholar]
- Drelicharz, L.; Kozlovski, V.; Skorka, T.; Heinze-Paluchowska, S.; Jasinski, A.; Gebska, A.; Guzik, T.; Olszanecki, R.; Wojnar, L.; Mende, U.; et al. No and pgi(2) in coronary endothelial dysfunction in transgenic mice with dilated cardiomyopathy. Basic Res. Cardiol 2008, 103, 417–430. [Google Scholar]
- Lorkowska, B.; Bartus, M.; Franczyk, M.; Kostogrys, R.B.; Jawien, J.; Pisulewski, P.M.; Chlopicki, S. Hypercholesterolemia does not alter endothelial function in spontaneously hypertensive rats. J. Pharmacol. Exp. Ther 2006, 317, 1019–1026. [Google Scholar]
- Koppenol, W.H.; Moreno, J.J.; Pryor, W.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol 1992, 5, 834–842. [Google Scholar]
- Pfeiffer, S.; Gorren, A.C.; Schmidt, K.; Werner, E.R.; Hansert, B.; Bohle, D.S.; Mayer, B. Metabolic fate of peroxynitrite in aqueous solution. Reaction with nitric oxide and ph-dependent decomposition to nitrite and oxygen in a 2:1 stoichiometry. J. Biol. Chem 1997, 272, 3465–3470. [Google Scholar]
- Ketsawatsakul, U.; Whiteman, M.; Halliwell, B. A reevaluation of the peroxynitrite scavenging activity of some dietary phenolics. Biochem. Biophys. Res. Commun 2000, 279, 692–699. [Google Scholar]
- Arteel, G.E.; Briviba, K.; Sies, H. Protection against peroxynitrite. FEBS Lett 1999, 445, 226–230. [Google Scholar]
- Kaur, I.P.; Geetha, T. Screening methods for antioxidants—A review. Mini Rev. Med. Chem 2006, 6, 305–312. [Google Scholar]
- Merenyi, G.; Lind, J.; Goldstein, S. The rate of homolysis of adducts of peroxynitrite to the C=O double bond. J. Am. Chem. Soc 2002, 124, 40–48. [Google Scholar]
- Sawa, T.; Akaike, T.; Maeda, H. Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J. Biol. Chem 2000, 275, 32467–32474. [Google Scholar]
- Boddupalli, S.S.; Estabrook, R.W.; Peterson, J.A. Fatty acid monooxygenation by cytochrome p-450bm-3. J. Biol. Chem 1990, 265, 4233–4239. [Google Scholar]
- Graham-Lorence, S.; Truan, G.; Peterson, J.A.; Falck, J.R.; Wei, S.; Helvig, C.; Capdevila, J.H. An active site substitution, f87v, converts cytochrome p450 bm-3 into a regio- and stereoselective (14s,15r)-arachidonic acid epoxygenase. J. Biol. Chem 1997, 272, 1127–1135. [Google Scholar]
- Jung, C.; Hoa, G.H.; Schroder, K.L.; Simon, M.; Doucet, J.P. Substrate analogue induced changes of the co-stretching mode in the cytochrome p450cam-carbon monoxide complex. Biochemistry 1992, 31, 12855–12862. [Google Scholar]
- Nakahara, K.; Tanimoto, T.; Hatano, K.; Usuda, K.; Shoun, H. Cytochrome p-450 55a1 (p-450dnir) acts as nitric oxide reductase employing nadh as the direct electron donor. J. Biol. Chem 1993, 268, 8350–8355. [Google Scholar]
- Wada, M.; Yokoyama, C.; Hatae, T.; Shimonishi, M.; Nakamura, M.; Imai, Y.; Ullrich, V.; Tanabe, T. Purification and characterization of recombinant human prostacyclin synthase. J. Biochem 2004, 135, 455–463. [Google Scholar]
- Lee, D.S.; Nioche, P.; Hamberg, M.; Raman, C.S. Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature 2008, 455, 363–368. [Google Scholar]
- Schuhmacher, S.; Oelze, M.; Bollmann, F.; Kleinert, H.; Otto, C.; Heeren, T.; Steven, S.; Hausding, M.; Knorr, M.; Pautz, A.; et al. Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy. Diabetes 2011, 60, 2608–2616. [Google Scholar]
- Oelze, M.; Knorr, M.; Schuhmacher, S.; Heeren, T.; Otto, C.; Schulz, E.; Reifenberg, K.; Wenzel, P.; Munzel, T.; Daiber, A. Vascular dysfunction in streptozotocin-induced experimental diabetes strictly depends on insulin deficiency. J. Vasc. Res 2011, 48, 275–284. [Google Scholar]
- Siegle, I.; Nusing, R.; Brugger, R.; Sprenger, R.; Zecher, R.; Ullrich, V. Characterization of monoclonal antibodies generated against bovine and porcine prostacyclin synthase and quantitation of bovine prostacyclin synthase. FEBS Lett 1994, 347, 221–225. [Google Scholar]
- Nathans, G.R.; Hade, E.P. Proteolytic activity in bovine milk xanthine oxidase preparations. Biochem. Biophys. Res. Commun 1975, 66, 108–114. [Google Scholar]
- Zou, M.H.; Shi, C.; Cohen, R.A. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin h(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes 2002, 51, 198–203. [Google Scholar]
- Zou, M.H.; Leist, M.; Ullrich, V. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am. J. Pathol 1999, 154, 1359–1365. [Google Scholar]
- Zou, M.H.; Bachschmid, M. Hypoxia-reoxygenation triggers coronary vasospasm in isolated bovine coronary arteries via tyrosine nitration of prostacyclin synthase. J. Exp. Med 1999, 190, 135–139. [Google Scholar]
- Hink, U.; Oelze, M.; Kolb, P.; Bachschmid, M.; Zou, M.H.; Daiber, A.; Mollnau, H.; August, M.; Baldus, S.; Tsilimingas, N.; et al. Role for peroxynitrite in the inhibition of prostacyclin synthase in nitrate tolerance. J. Am. Coll. Cardiol 2003, 42, 1826–1834. [Google Scholar]
- Bachschmid, M.; Thurau, S.; Zou, M.H.; Ullrich, V. Endothelial cell activation by endotoxin involves superoxide/no-mediated nitration of prostacyclin synthase and thromboxane receptor stimulation. FASEB J 2003, 17, 914–916. [Google Scholar]
| Scavenger | IC50 (μM) | Scavenger | IC50 (μM) |
|---|---|---|---|
| Glutathione | 181 ± 20/380 ± 57 | Methionine | 450 ± 34/690 ± 75 |
| Ascorbate | 88 ± 18/133 ± 18 | Uric acid | 40 ± 10/57 ± 8 |
| 2,6-Dithiopurine | 35 ± 13/36 ± 14 | 2,6-Dithiopyrimidine | 32 ± 4/43.5 ± 9.5 |
| Ebselen | 128 ± 5/190 ± 11 | 1,3-Dimethyluric acid | 36.5 ± 1.5/25 ± 12 |
| Se-methionine | 250 ± 13/170 ± 28 | Xanthine | >1 mM/>1 mM |
| Cysteine | 64 ± 14/425 ± 125 | 3,9-Dimethyluric acid | 141 ± 7/n.d. |
| Alloxan | >1 mM/n.d. | 3,7-Dimethyluric acid | 19 ± 3/n.d. |
| 2-Thiobarbituric acid | 37 ± 6/26 ± 4 | Allopurinol | >1 mM/>1 mM |
| Caffeine | >1 mM/n.d. | Allantoin | >1 mM/n.d. |
| Scavenger | IC50 (μM) | Scavenger | IC50 (μM) |
|---|---|---|---|
| Glutathione | 150 ± 26 | Methionine | 200 ± 38 |
| Ascorbate | 170 ± 17 | Uric acid | 75 ± 13 |
| 2-Thiobarbituric acid | 45 ± 12 | 1,3-Dimethyluric acid | 60 ± 14 |
| Ebselen | 180 ± 12 | 3,7-Dimethyluric acid | 185 ± 37 |
| Xanthine | >500 | 3,9-Dimethyluric acid | >200 |
| Allopurinol | >500 | - | - |
| Scavenger | IC50 (μM) | Scavenger | IC50 (μM) |
|---|---|---|---|
| Glutathione | 31 ± 2.5 | Methionine | 185 ± 7 |
| Ascorbate | *** | Uric acid | 400/26 ± 2 * |
| 2,6-Dithiopurine | 40 ± 5 ** | 2,6-Dithiopyrimidine | 45 ± 8 ** |
| Ebselen | *** | 3,7-Dimethyluric acid | 400/42 ± 1.5 * |
| Se-methionine | 15 ± 1 | Tryptophan | 400/35 ± 3 * |
| Cysteine | 18 ± 3 | Tyrosine | 420 ± 30 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Daiber, A.; Daub, S.; Bachschmid, M.; Schildknecht, S.; Oelze, M.; Steven, S.; Schmidt, P.; Megner, A.; Wada, M.; Tanabe, T.; et al. Protein Tyrosine Nitration and Thiol Oxidation by Peroxynitrite—Strategies to Prevent These Oxidative Modifications. Int. J. Mol. Sci. 2013, 14, 7542-7570. https://doi.org/10.3390/ijms14047542
Daiber A, Daub S, Bachschmid M, Schildknecht S, Oelze M, Steven S, Schmidt P, Megner A, Wada M, Tanabe T, et al. Protein Tyrosine Nitration and Thiol Oxidation by Peroxynitrite—Strategies to Prevent These Oxidative Modifications. International Journal of Molecular Sciences. 2013; 14(4):7542-7570. https://doi.org/10.3390/ijms14047542
Chicago/Turabian StyleDaiber, Andreas, Steffen Daub, Markus Bachschmid, Stefan Schildknecht, Matthias Oelze, Sebastian Steven, Patrick Schmidt, Alexandra Megner, Masayuki Wada, Tadashi Tanabe, and et al. 2013. "Protein Tyrosine Nitration and Thiol Oxidation by Peroxynitrite—Strategies to Prevent These Oxidative Modifications" International Journal of Molecular Sciences 14, no. 4: 7542-7570. https://doi.org/10.3390/ijms14047542






