Influence of Growth Conditions on Magnetite Nanoparticles Electro-Crystallized in the Presence of Organic Molecules
Abstract
:1. Introduction
2. Results and Discussion
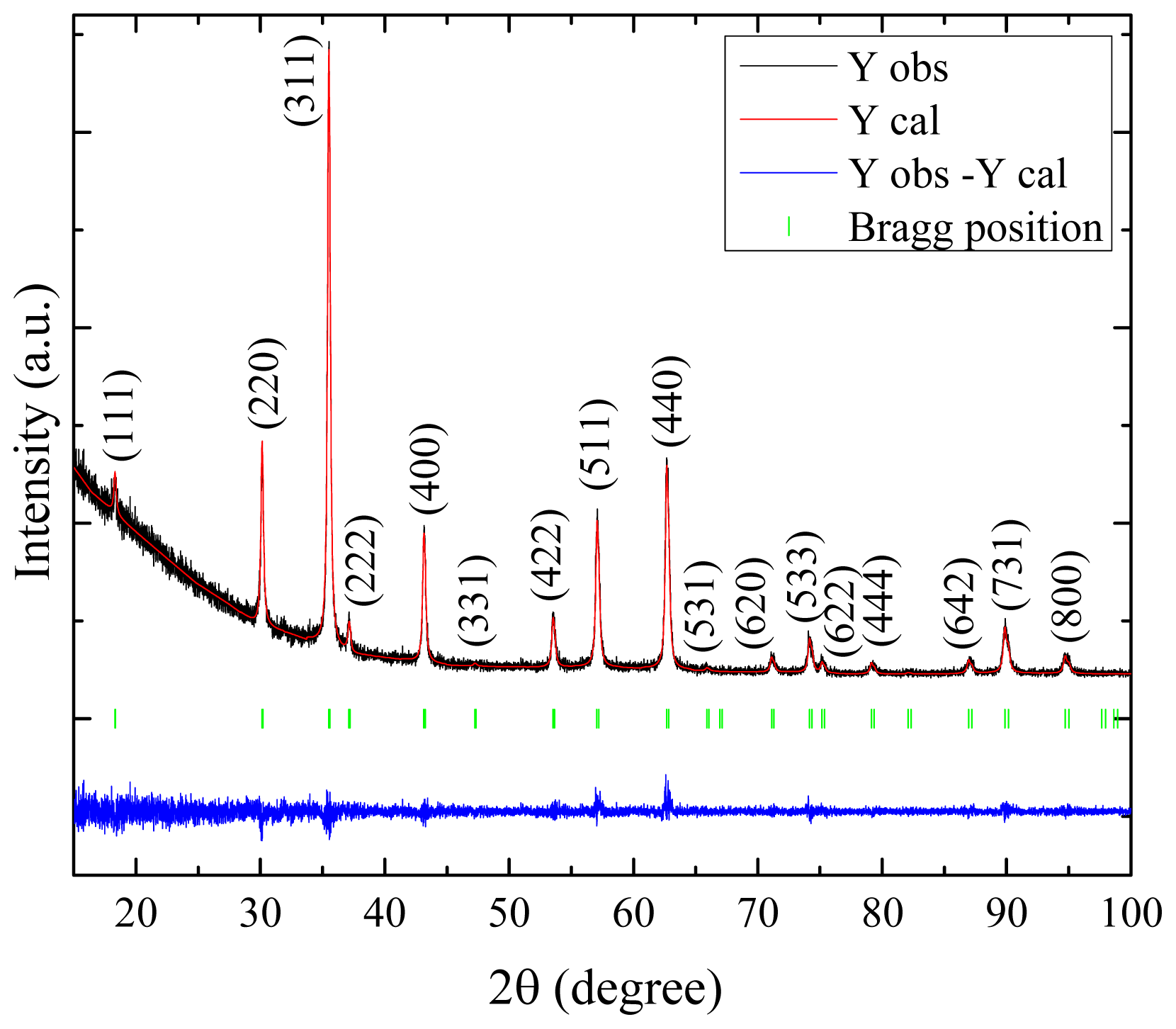
2.1. XRD Results
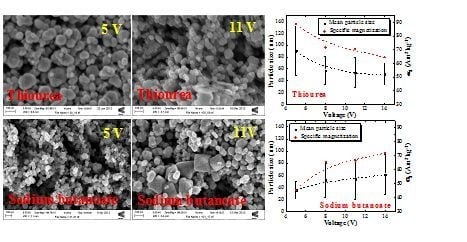
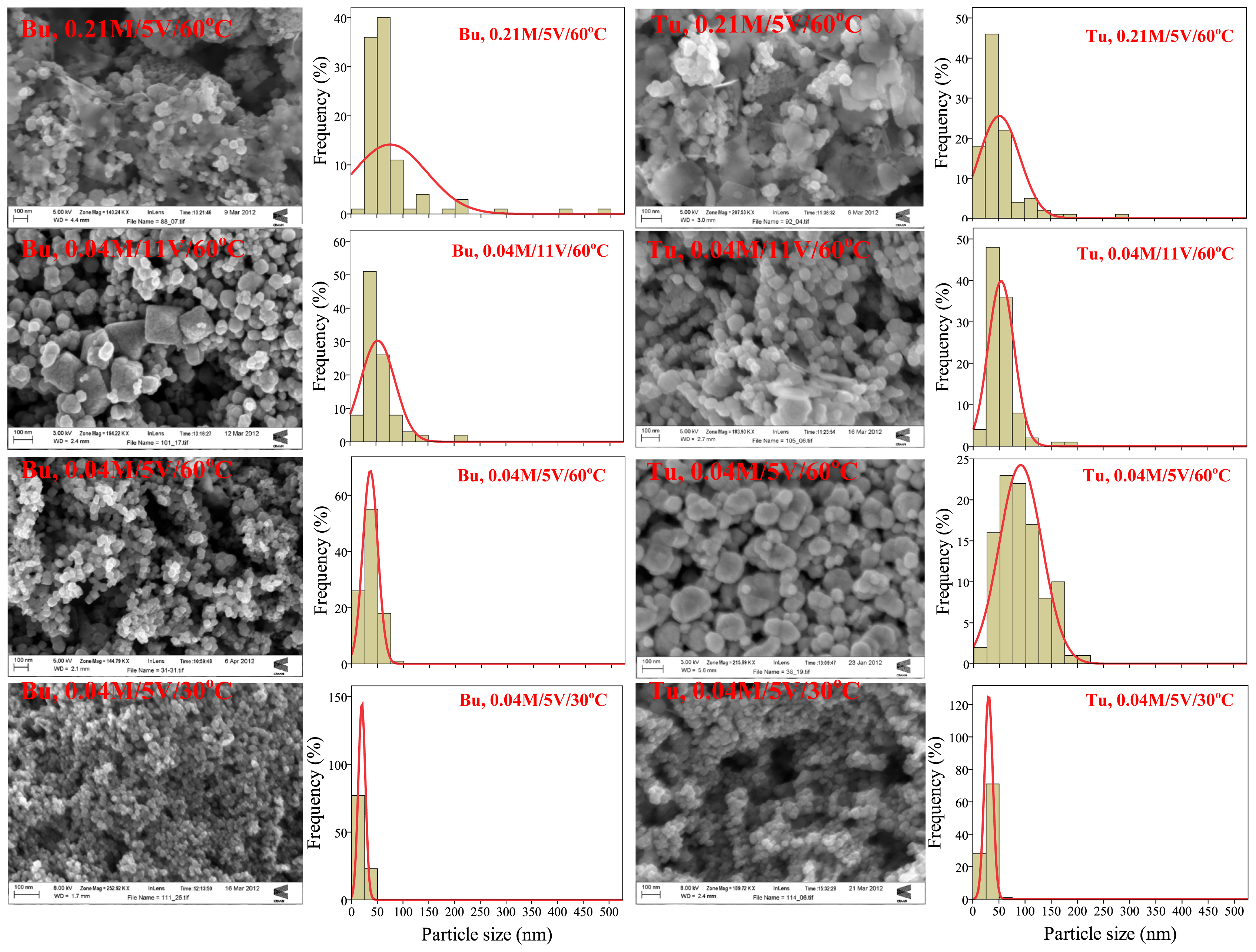
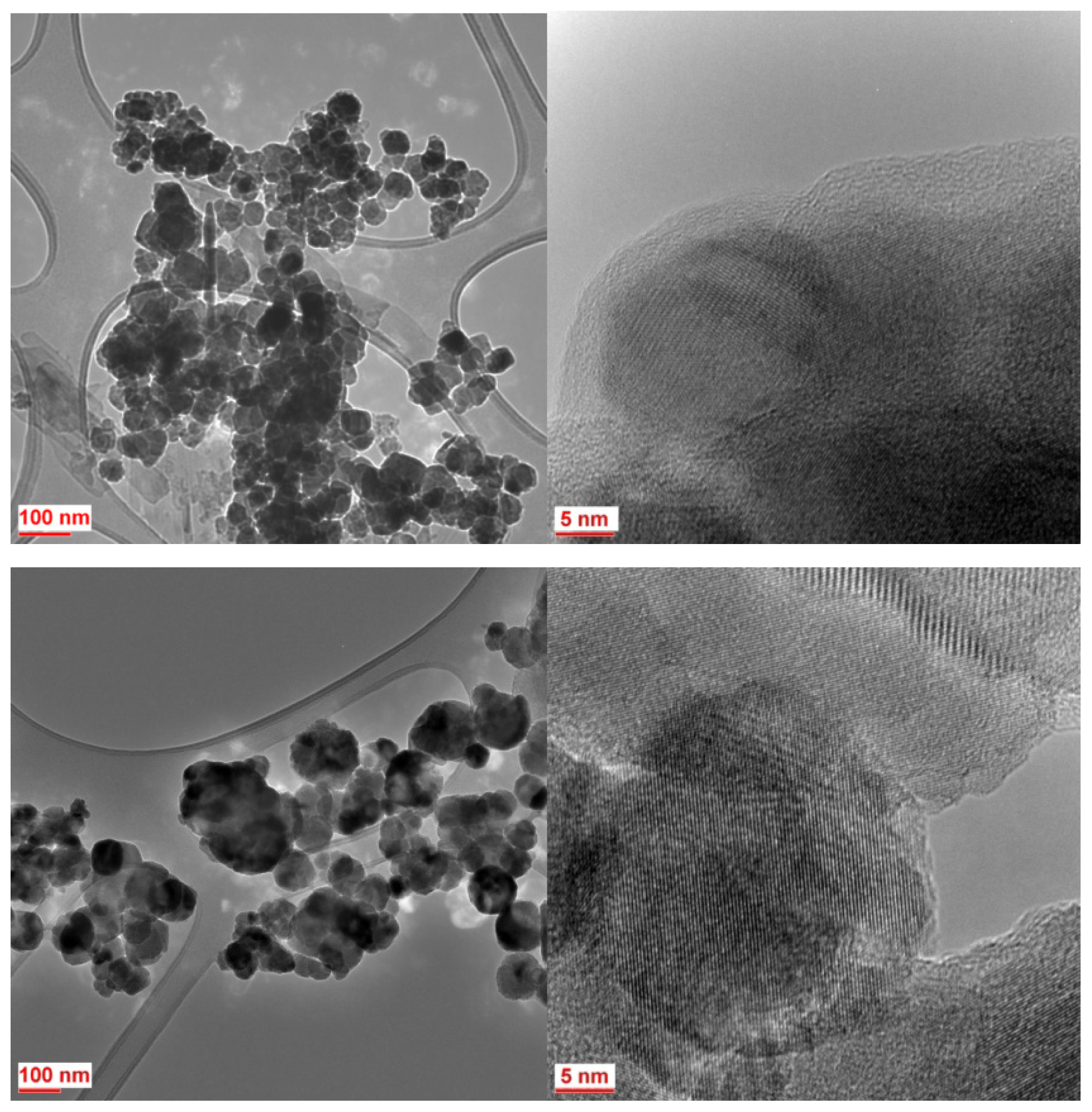
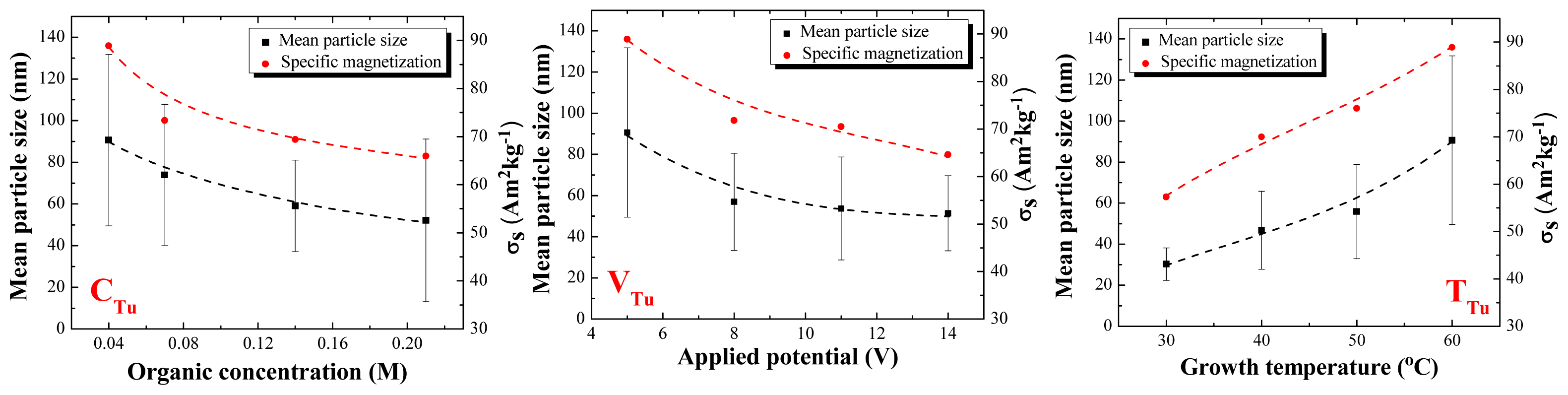
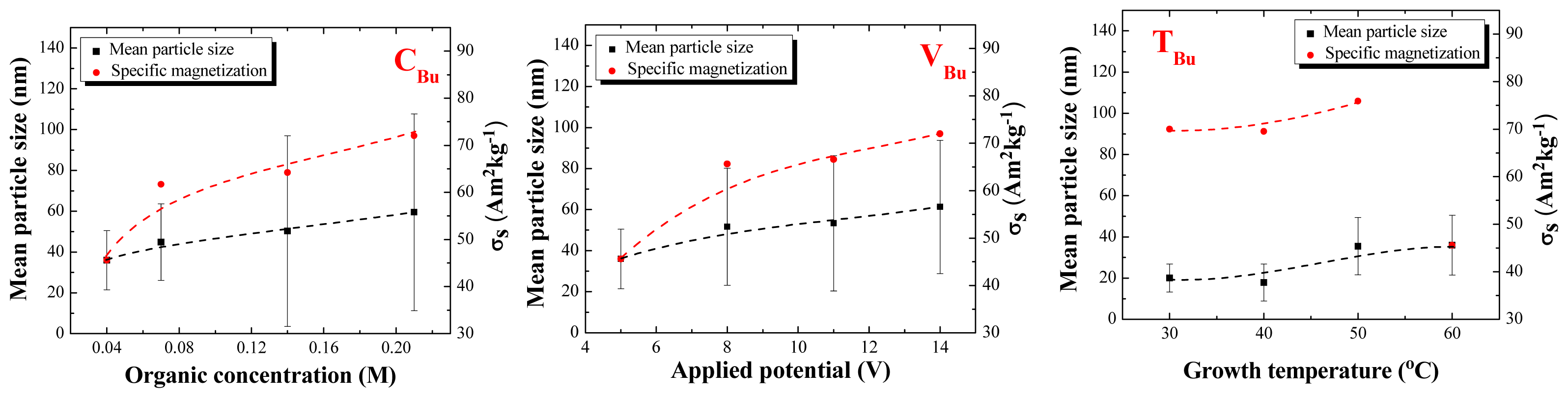
2.2. Particle Size Distribution and Morphology
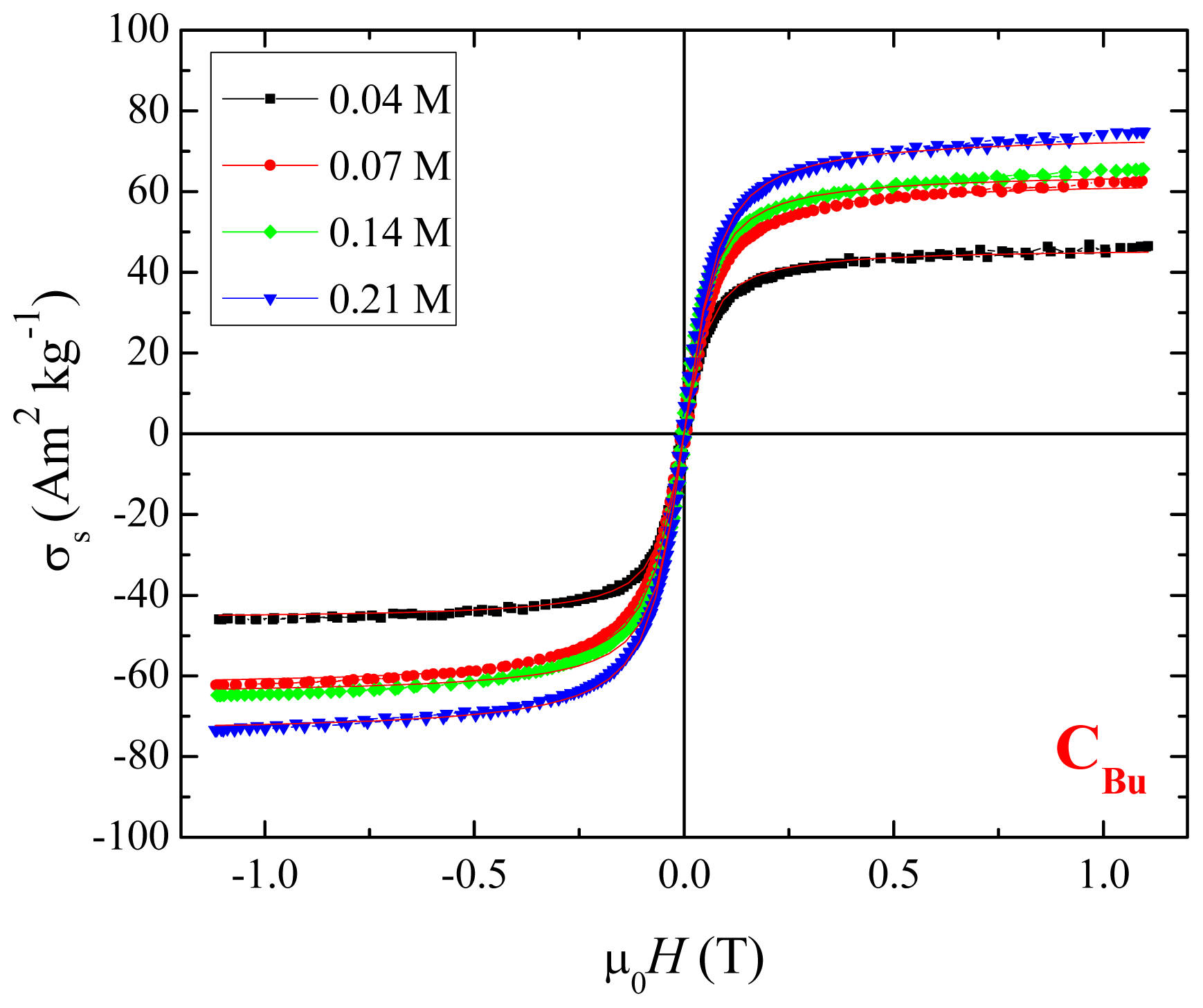
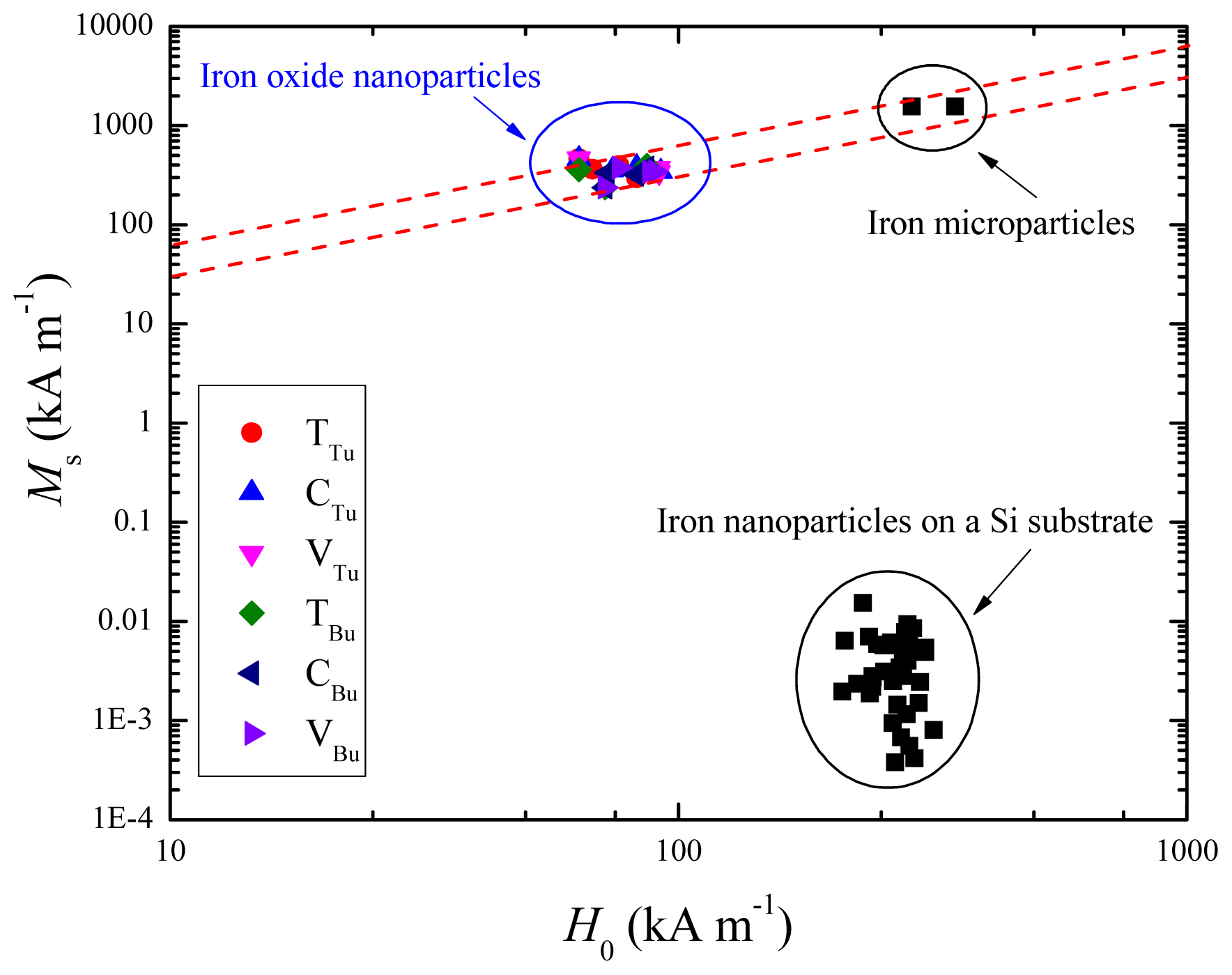
2.3. Magnetic Properties
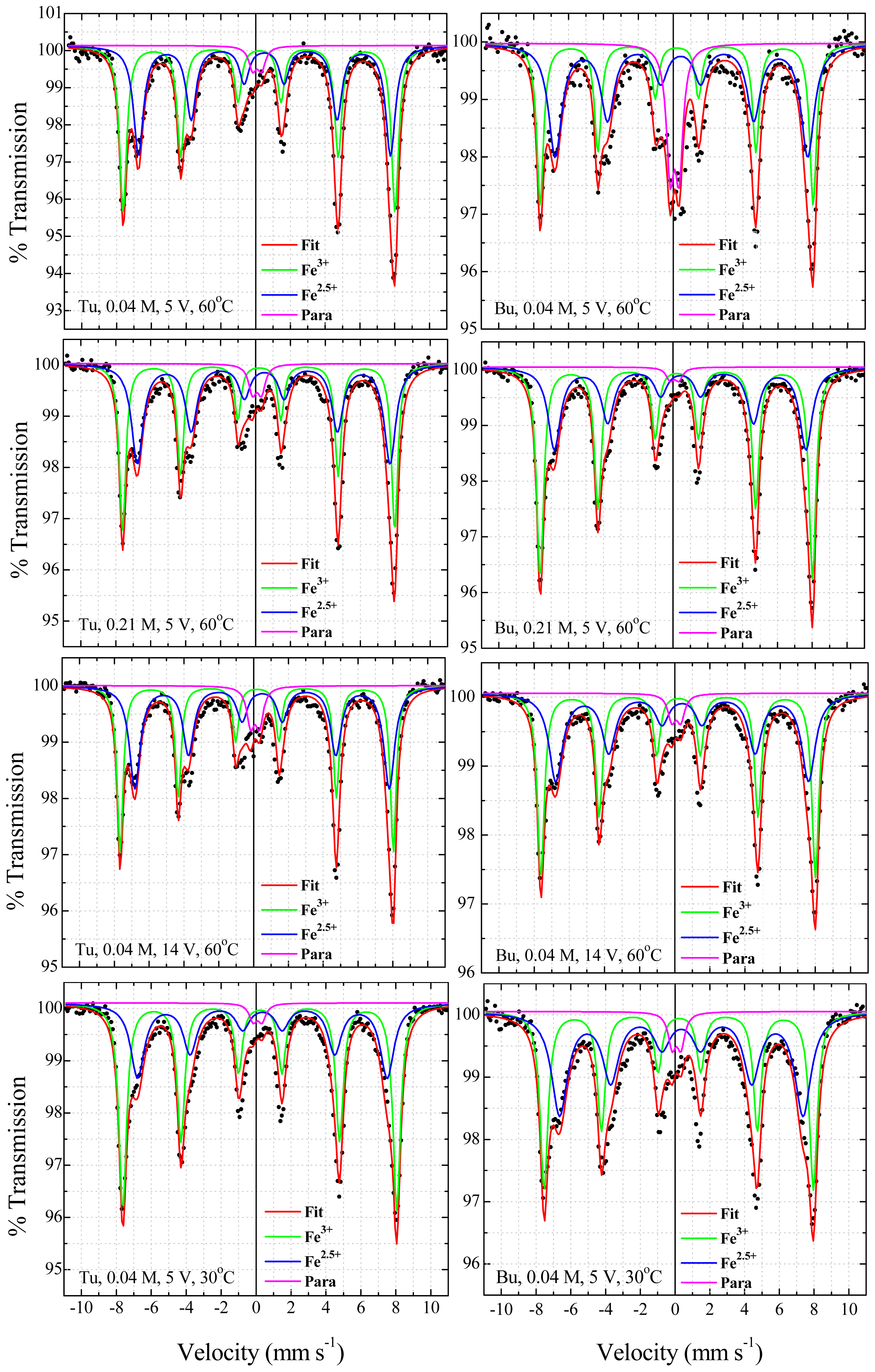
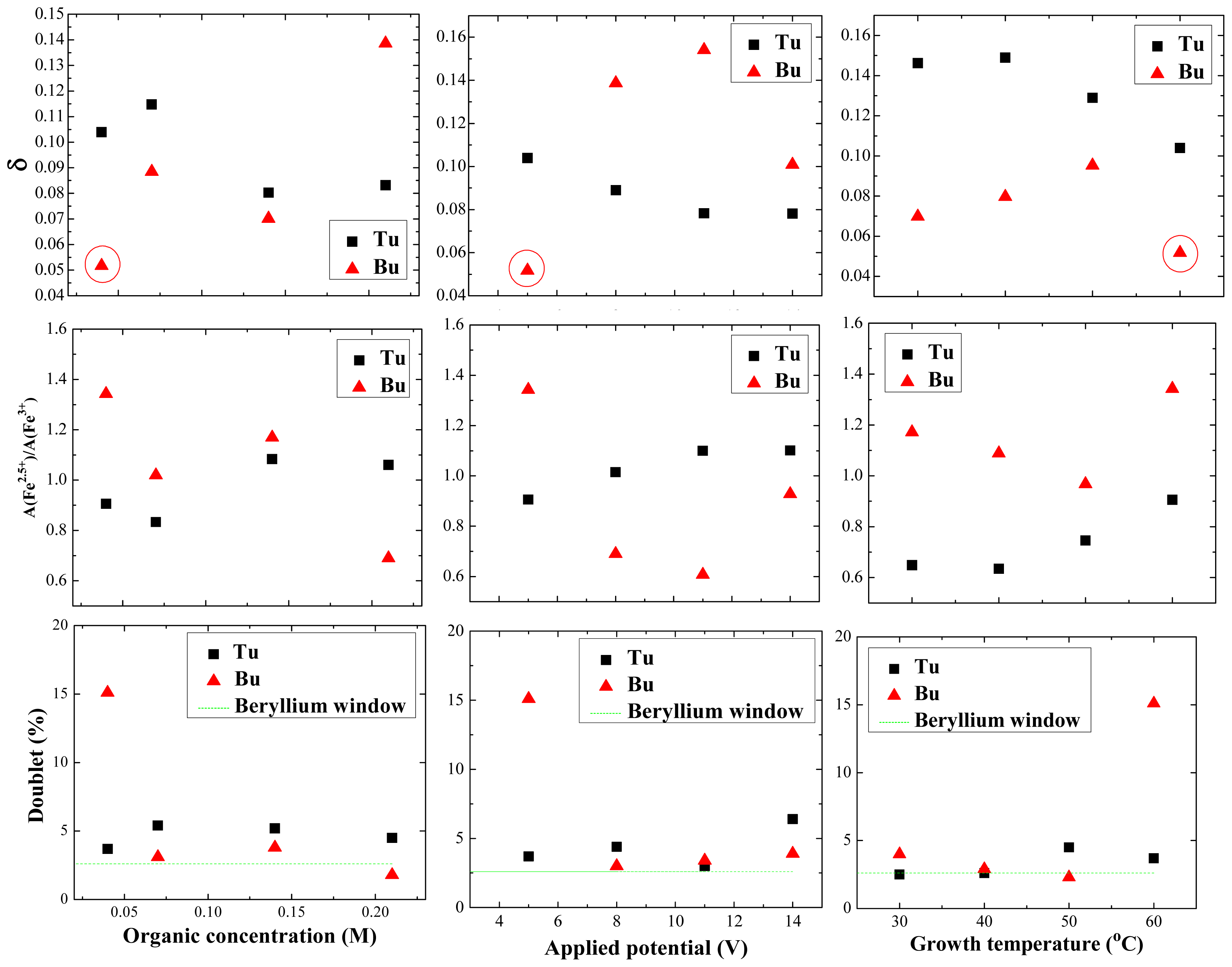
2.4. Mössbauer Spectroscopy
3. Experimental Section
3.1. Materials
3.2. Methods
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Mørup, S.; Hansen, M.F.; Frandsen, C. Comprehensive Nanoscience and Technology; Oxford; Academic Press: Kongens Lyngby, Denmark, 2011; Volume 1, Chapter 14, pp. 437–491. [Google Scholar]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D 2009, 42, 1–15. [Google Scholar]
- Prina-Mello, A.; Whelan, A.M.; Atzberger, A.; McCarthy, J.E.; Byrne, F.; Davies, G.L.; Coey, J.M.D.; Volkov, Y.; Guńko, Y.K. Comparative flow cytometric analysis of amino functionalized nanowire and nanoparticle signatures. Small 2010, 6, 247–255. [Google Scholar]
- Probst, C.E.; Zrazhevskiy, P.; Gao, X. Rapid multitarget immunomagnetic separation through programmable DNA linker displacement. J. Am. Chem. Soc 2011, 133, 17126–17129. [Google Scholar]
- Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. Nat. Nanotechnol 2008, 3, 139–143. [Google Scholar]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater 2005, 4, 435–446. [Google Scholar]
- Luque, G.L.; Ferreyra, N.F.; Leyva, G.; Rivas, G.A. Characterization of carbon paste electrodes modified with manganese based perovskites-type oxides from the amperometric determination of hydrogen peroxide. Sens. Actuators B 2009, 142, 331–336. [Google Scholar]
- Comba, F.N.; Gutierrez, F.; Herrasti, P.; Rubianes, M.D.; Rivas, G.A. Effect of the incorporation of proteins on the performance of carbon paste electrodes modified with electrogenerated magnetite nanoparticles towards the reduction of hydrogen peroxide. Electroanalysis 2012, 24, 1541–1546. [Google Scholar]
- Li, J.; Quia, X.; Lin, Y.; Liu, X.; Gao, R.; Wang, A. A study of modified Fe3O4 nanoparticles for the synthesis of ionic ferrofluid. Appl. Surf. Sci 2010, 256, 6977–6981. [Google Scholar]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Delivery Rev 2011, 63, 789–808. [Google Scholar]
- Qu, J.; Liu, G.; Wang, Y.; Hong, R. Preparation of Fe3O4-chitosan nanoparticles used for hyperthermia. Adv. Powder Technol 2010, 21, 461–467. [Google Scholar]
- Basti, H.; Ben Tahar, L.; Smiri, L.S.; Herbst, F.; Vaulay, M.-J.; Chau, F.; Ammar, S.; Benderbous, S. Catechol derivatives-coated Fe3O4 and γ-Fe2O3 nanoparticles as potential MRI contrast agents. J. Colloid Interface Sci 2010, 341, 248–254. [Google Scholar]
- Mohapatra, S.; Mallick, S.K.; Maiti, T.K.; Ghosh, S.K.; Pramanik, P. Synthesis of highly stable folic acid conjugated magnetite nanoparticles for targeting cancer cells. Nanotechnology 2007, 18, 385102. [Google Scholar]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ 2012, 424, 1–10. [Google Scholar]
- Wu, Y.; Zhang, J.; Tong, Y.; Xu, X. Chromium (VI) reduction in aqueous solutions by Fe3O4-stabilized Fe° nanoparticles. J. Hazard. Mater 2009, 172, 1640–1645. [Google Scholar]
- Bedanta, S.; Kleemann, W. Supermagnetism. J. Phys. D 2009, 42, 1–28. [Google Scholar]
- Gubin, S.P.; Koksharov, Y.A.; Khomutov, G.B.; Yurkov, G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev 2005, 74, 489–520. [Google Scholar]
- Indira, T.K.; Lakshmi, P.K. Magnetic nanoparticles—A review. Int. J. Pharm. Sci. Nanotechnol 2010, 3, 1035–1042. [Google Scholar]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev 2009, 38, 2532–2542. [Google Scholar]
- Amara, D.; Felner, I.; Nowik, I.; Margel, S. Synthesis and characterization of Fe and Fe3O4 nanoparticles by thermal decomposition of tri-iron dodecacarbonyl. Colloids Surf. A 2009, 339, 106–110. [Google Scholar]
- Liu, J.; Sun, B.; Hu, J.; Pei, Y.; Li, H.; Qiao, M. Aqueous-phase reforming of ethylene glycol to hydrogen on Pd/Fe3O4 catalyst prepared by co-precipitation: Metal-support interaction and excellent intrinsic activity. J. Catal 2010, 274, 287–295. [Google Scholar]
- Liu, Y.; Liu, P.; Su, Z.; Li, F.; Wen, F. Attapulgite-Fe3O4 magnetic nanoparticles via co-precipitation technique. Appl. Surf. Sci 2008, 255, 2020–2025. [Google Scholar]
- Chen, D.; Ni, S.; Chen, Z. Synthesis of Fe3O4 nanoparticles by wet milling iron powder in a planetary ball mill. China Particuol 2007, 5, 357–358. [Google Scholar]
- Gao, G.; Huang, P.; Zhang, Y.; Wang, K.; Qin, W.; Cui, D. Gram scale synthesis of superparamagnetic Fe3O4 nanoparticles and fluid via a facile solvothermal route. CrystEngComm 2011, 13, 1782–1785. [Google Scholar]
- Wei, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett 2008, 3, 397–415. [Google Scholar]
- Cabrera, L.; Gutierrez, S.; Menendez, N.; Morales, M.P.; Herrasti, P. Magnetite nanoparticles: Electrochemical synthesis and characterization. Electrochim. Acta 2008, 53, 3436–3441. [Google Scholar]
- Kazeminezhad, I.; Mosivand, S.; Farbod, M. Effect of growth parameters on structure of electrooxidized Fe3O4 magnetic nanoparticles. Curr. Nanosci 2011, 7, 819–824. [Google Scholar]
- Cabrera, L.I.; Martínez, M.; Reyman, D.; Crespo, P.; Morales, M.P.; Herrasti, P. One single-step synthesis of multifunctional methyleneblue-coated magnetite nanoparticles. J. Nanopart. Res 2011, 13, 6931–6939. [Google Scholar]
- Kazeminezhad, I.; Mosivand, S. Effect of surfactant concentration on size and morphology of sonoelectrooxidized Fe3O4 nanoparticles. Curr. Nanosci 2012, 8, 623–627. [Google Scholar]
- Mosivand, S.; Monzon, L.M.A.; Ackland, K.; Kazeminezhad, I.; Coey, J.M.D. The effect of organics on the structure and magnetization of electro-synthesised magnetite nanoparticles. J. Nanopart. Res. 2013. submitted for publication. [Google Scholar]
- Abdulwahab, K.; Malik, M.A.; O’Brien, P.; Govender, K.; Muryn, C.A.; Timco, G.A.; Tuna, F.; Winpenny, R.E.P. Synthesis of monodispersed magnetite nanoparticles from iron pivalate clusters. Dalton Trans 2013, 42, 196–206. [Google Scholar]
- Coey, J.M.D.; Mlack, J.T.; Venkatesan, M.; Stamenov, P. Magnetization process in dilute magnetic oxides. IEEE Trans. Magn 2010, 46, 2501–2503. [Google Scholar]
- Grace, P.J.; Venkatesan, M.; Alaria, J.; Coey, J.M.D.; Kopnov, G.; Naaman, R. The origin of the magnetism of etched silicon. Adv. Mater 2009, 21, 71–74. [Google Scholar]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010; pp. 243–244. [Google Scholar]
- Coey, J.M.D.; Morrish, A.H.; Sawatzky, G.A. A Mössbauer study of conduction in magnetite. J. Phys. Colloques 1971, 32, C1, :271–C1:273.. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mosivand, S.; Monzon, L.M.A.; Kazeminezhad, I.; Coey, J.M.D. Influence of Growth Conditions on Magnetite Nanoparticles Electro-Crystallized in the Presence of Organic Molecules. Int. J. Mol. Sci. 2013, 14, 10383-10396. https://doi.org/10.3390/ijms140510383
Mosivand S, Monzon LMA, Kazeminezhad I, Coey JMD. Influence of Growth Conditions on Magnetite Nanoparticles Electro-Crystallized in the Presence of Organic Molecules. International Journal of Molecular Sciences. 2013; 14(5):10383-10396. https://doi.org/10.3390/ijms140510383
Chicago/Turabian StyleMosivand, Saba, Lorena M. A. Monzon, Iraj Kazeminezhad, and J. Michael D. Coey. 2013. "Influence of Growth Conditions on Magnetite Nanoparticles Electro-Crystallized in the Presence of Organic Molecules" International Journal of Molecular Sciences 14, no. 5: 10383-10396. https://doi.org/10.3390/ijms140510383