The Role of Peritoneal Alternatively Activated Macrophages in the Process of Peritoneal Fibrosis Related to Peritoneal Dialysis
Abstract
:1. Introduction
2. Results
2.1. Alteration of Peritoneum in Peritoneal Fibrosis Mice
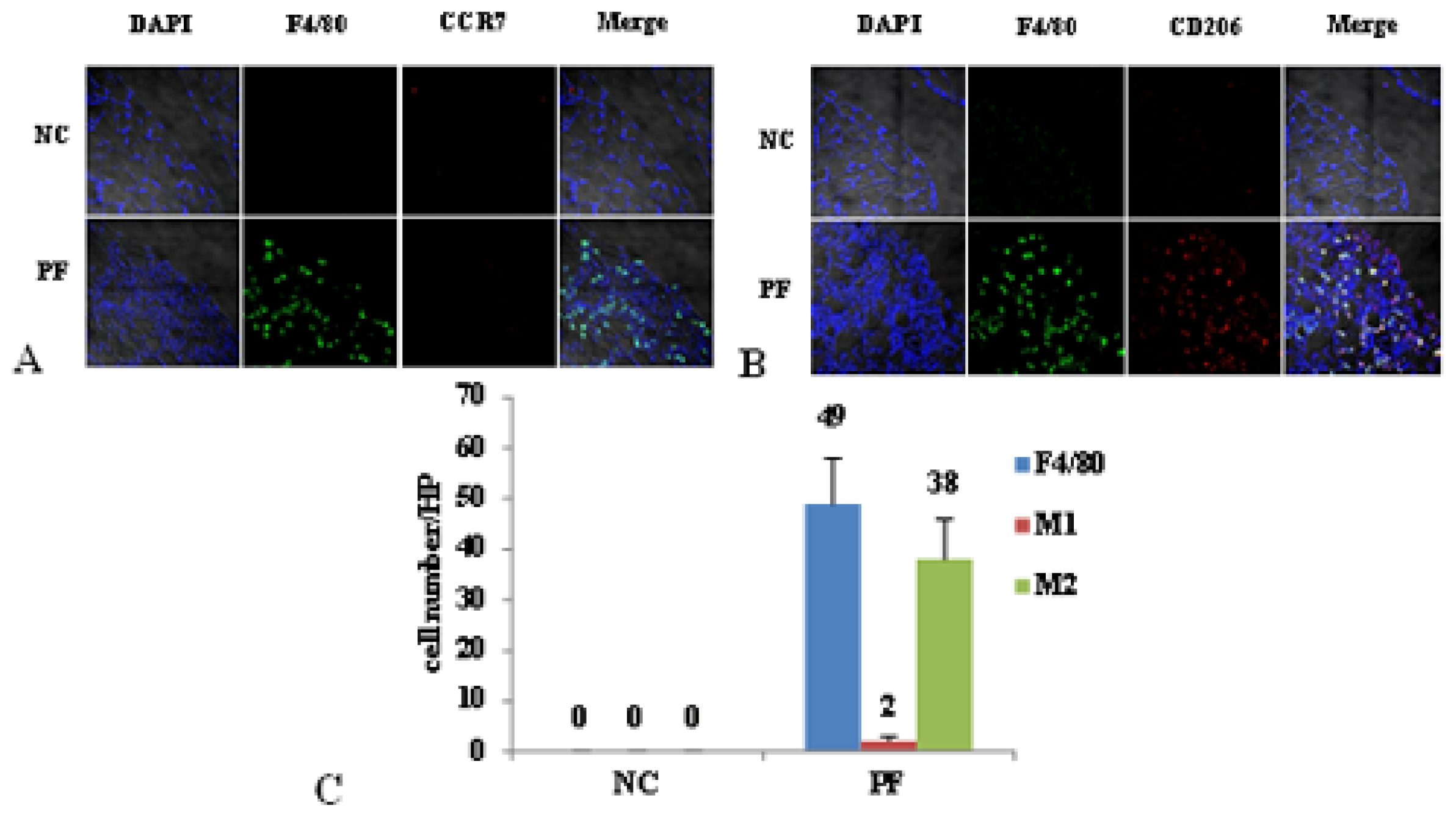
2.2. Macrophage Polarization in PF Mice
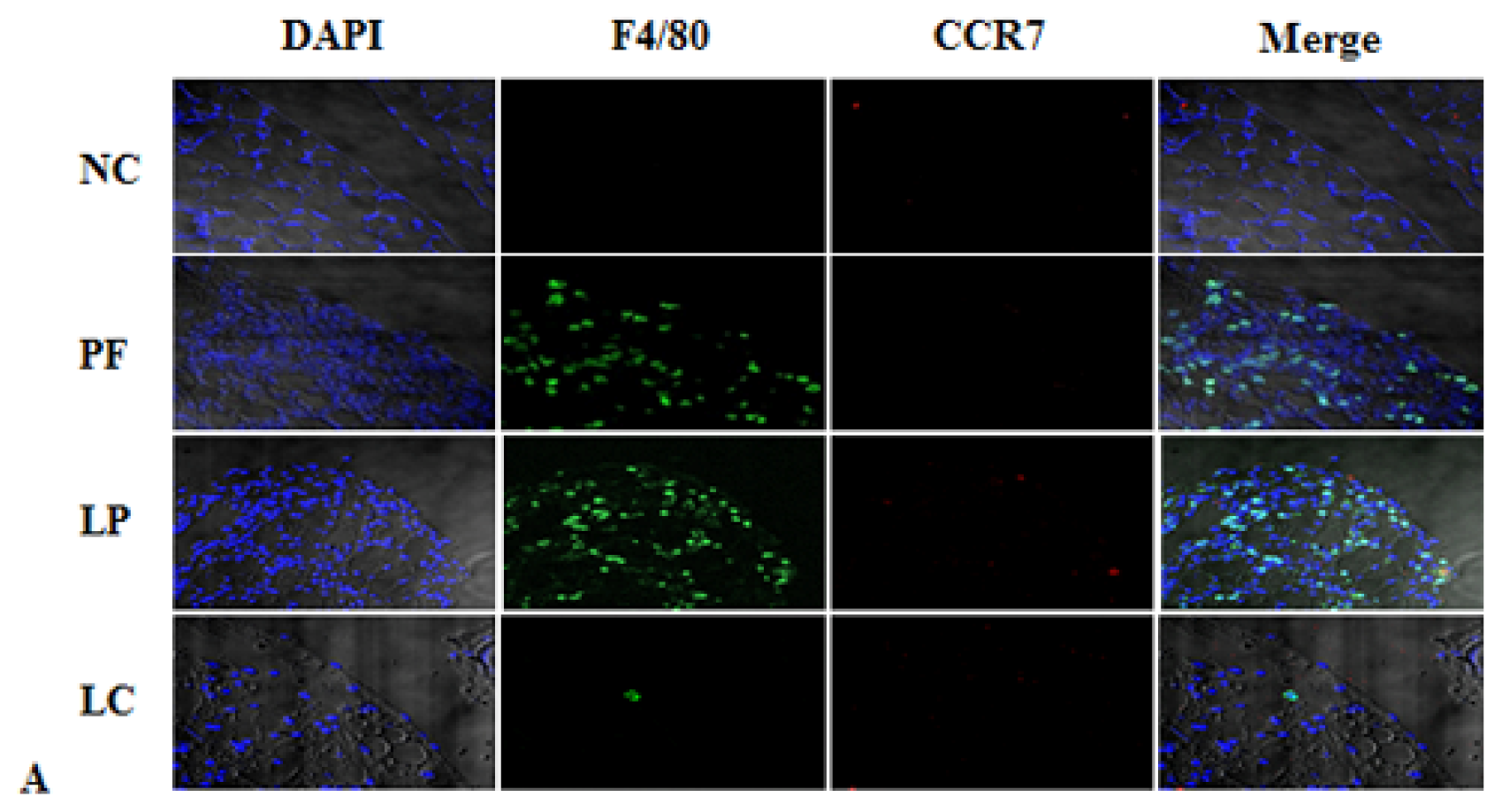
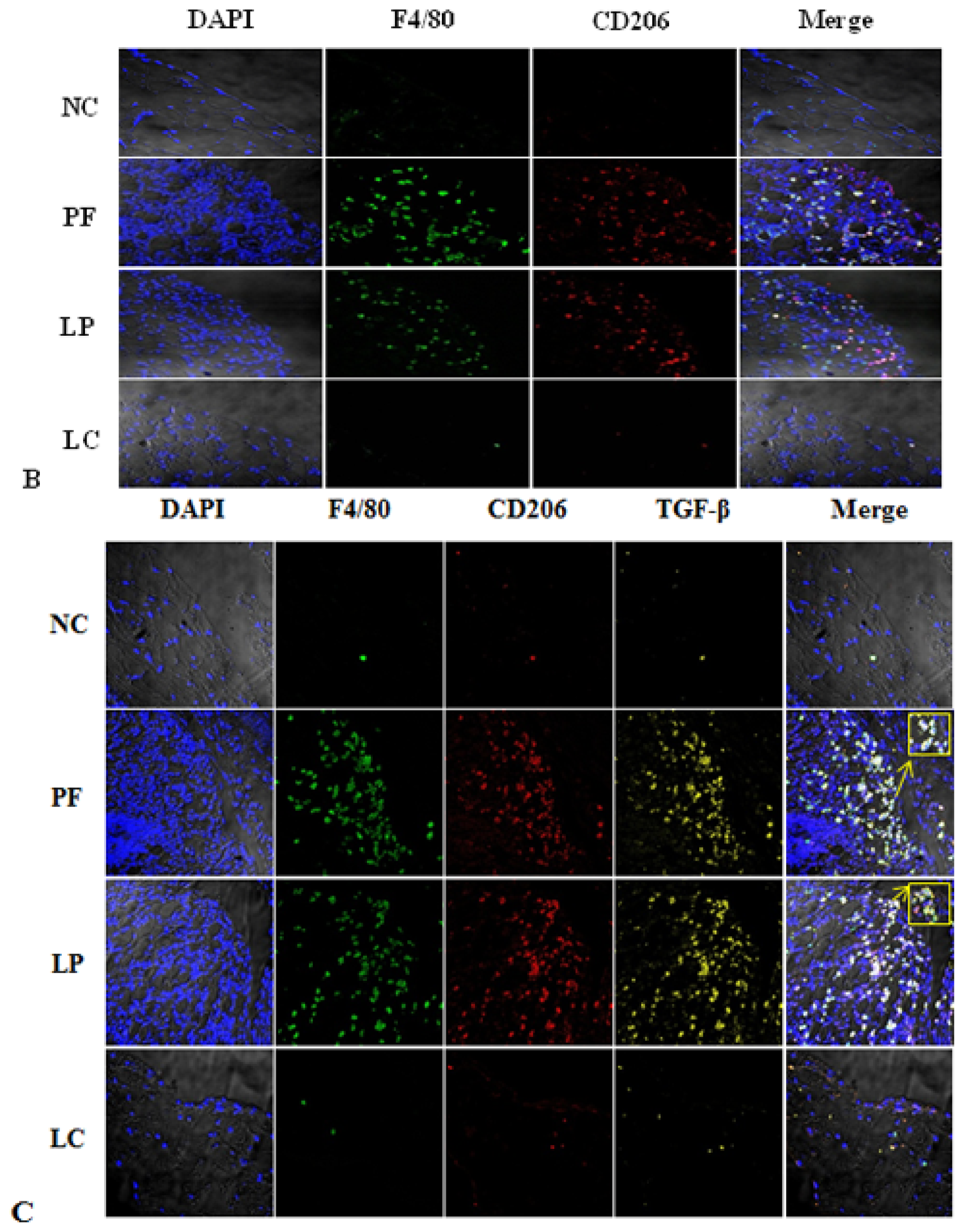
2.3. Effect of Liposome-Encapsulated Clodronate (LC) on Peritoneal M1 and M2
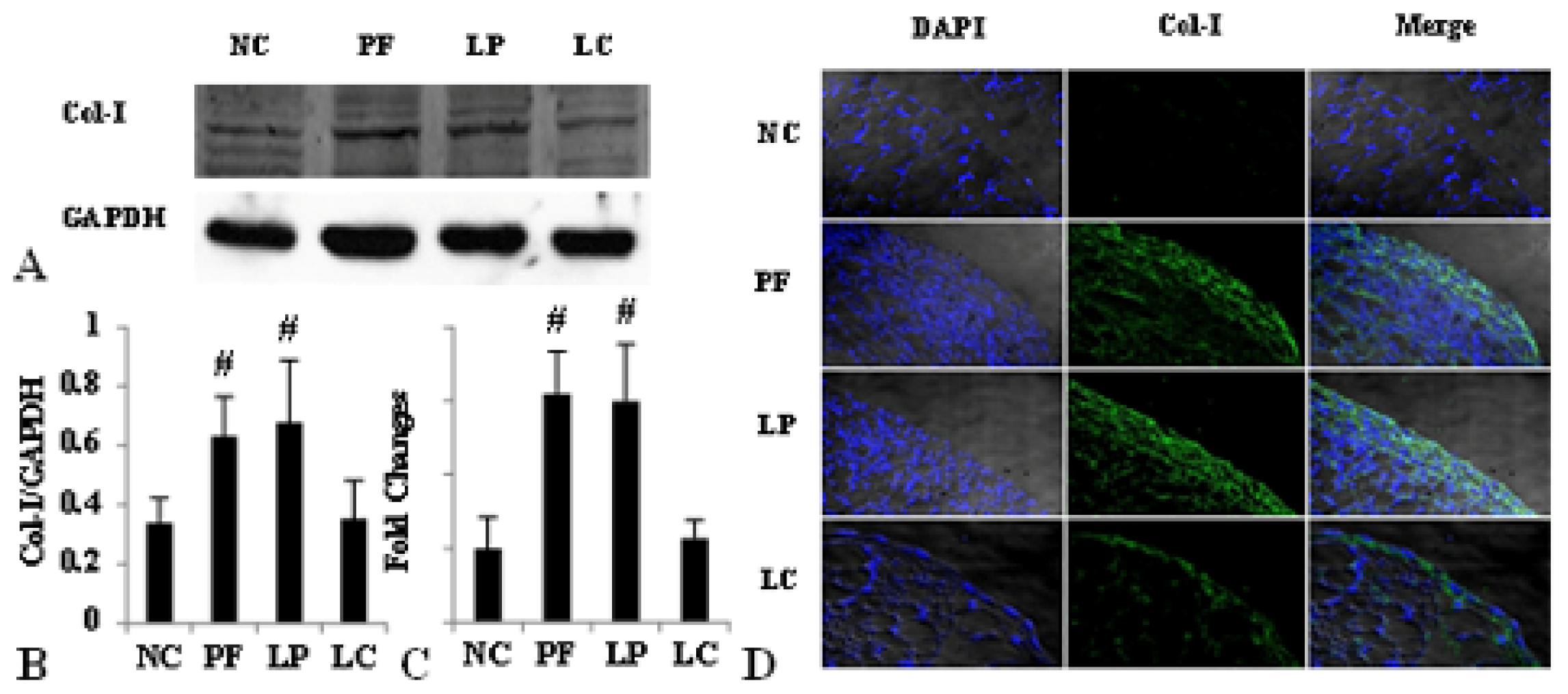
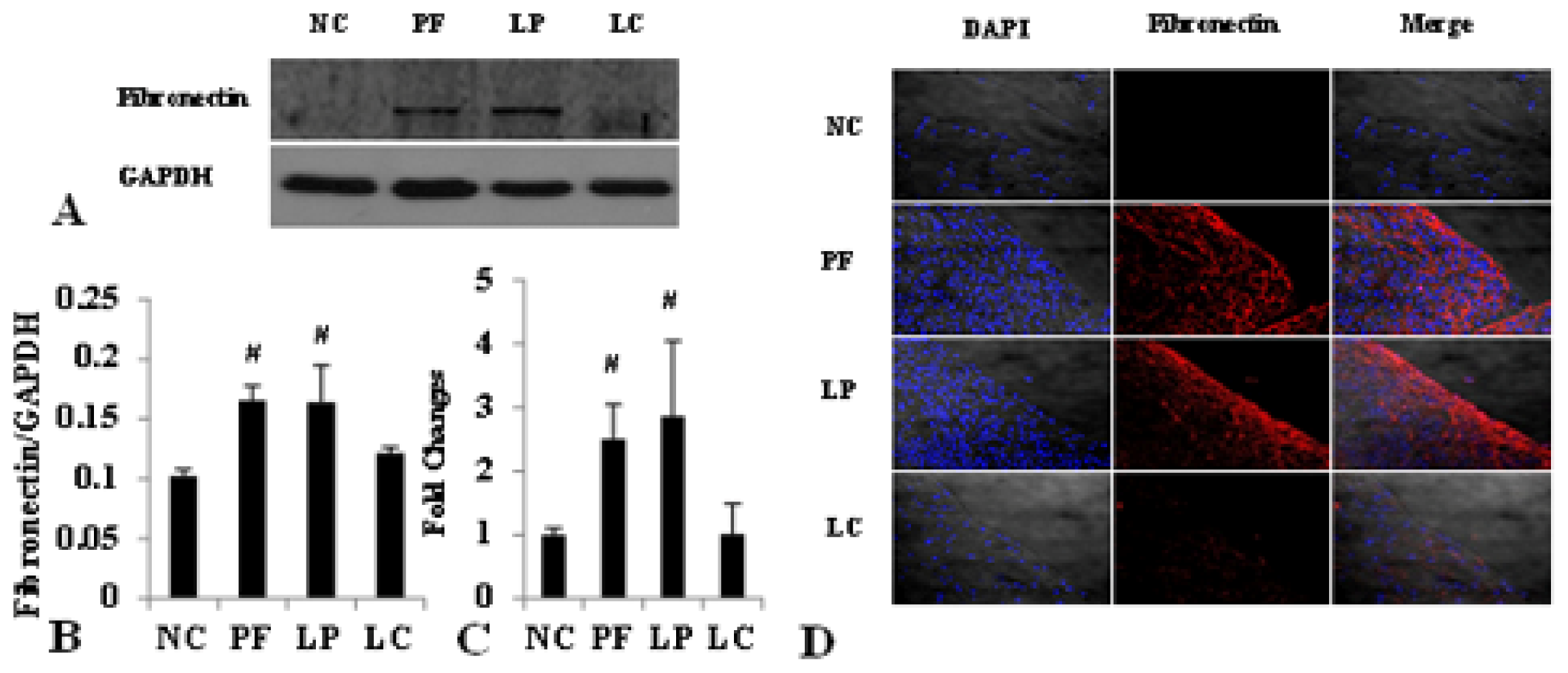
2.4. LC mitigated Peritoneal Fibrosis Related to PD by Depletion of M2 in Peritoneum
3. Discussion
4. Materials and Methods
4.1. Preparation of Liposome-Encapsulated Clodronate and Liposome-Encapsulated Phosphate Buffer
4.2. Animal Model and Tissue Preparation
4.3. Histology
4.4. Immunofluorescence
4.5. Cell Count
4.6. Western Blotting
4.7. Quantitative Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Grassmann, A.; Gioberge, S.; Moeller, S.; Brown, G. ESRD patients in 2004: Global overview of patient numbers, treatment modalities and associated trends. Nephrol. Dial. Transplant 2005, 20, 2587–2593. [Google Scholar]
- Aroeira, L.S.; Aguilera, A.; Sanchez-Tomero, J.A.; Bajo, M.A.; Del, P.G.; Jimenez-Heffernan, J.A.; Selgas, R.; Lopez-Cabrera, M. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J. Am. Soc. Nephrol 2007, 18, 2004–2013. [Google Scholar]
- Krediet, R.T.; Lindholm, B.; Rippe, B. Pathophysiology of peritoneal membrane failure. Perit Dial. Int 2000, 20, S22–S42. [Google Scholar]
- Yung, S.; Chan, T.M. Pathophysiological changes to the peritoneal membrane during PD-related peritonitis: The role of mesothelial cells. Mediat. Inflamm 2012, 2012, 484167. [Google Scholar]
- Di Paolo, N.; Sacchi, G. Atlas of peritoneal histology. Perit Dial. Int 2000, 20, S5–S96. [Google Scholar]
- Williams, J.D.; Craig, K.J.; Topley, N.; von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol 2002, 13, 470–479. [Google Scholar]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol 2008, 8, 958–969. [Google Scholar]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol 2003, 3, 23–35. [Google Scholar]
- Jaguin, M.; Houlbert, N.; Fardel, O.; Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol 2013, 281, 51–61. [Google Scholar]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol 2006, 177, 7303–7311. [Google Scholar]
- Badylak, S.F.; Valentin, J.E.; Ravindra, A.K.; Mccabe, G.P.; Stewart-Akers, A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng. A 2008, 14, 1835–1842. [Google Scholar]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol 2009, 27, 451–483. [Google Scholar]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med 1992, 176, 287–292. [Google Scholar]
- Murphy, B.S.; Bush, H.M.; Sundareshan, V.; Davis, C.; Hagadone, J.; Cory, T.J.; Hoy, H.; Hayes, D.J.; Anstead, M.I.; Feola, D.J. Characterization of macrophage activation states in patients with cystic fibrosis. J. Cyst. Fibros 2010, 9, 314–322. [Google Scholar]
- Higashi-Kuwata, N.; Jinnin, M.; Makino, T.; Fukushima, S.; Inoue, Y.; Muchemwa, F.C.; Yonemura, Y.; Komohara, Y.; Takeya, M.; Mitsuya, H.; et al. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res. Ther 2010, 12, R128. [Google Scholar]
- Murray, L.A.; Rosada, R.; Moreira, A.P.; Joshi, A.; Kramer, M.S.; Hesson, D.P.; Argentieri, R.L.; Mathai, S.; Gulati, M.; Herzog, E.L.; et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One 2010, 5, e9683. [Google Scholar]
- Mathai, S.K.; Gulati, M.; Peng, X.; Russell, T.R.; Shaw, A.C.; Rubinowitz, A.N.; Murray, L.A.; Siner, J.M.; Antin-Ozerkis, D.E.; Montgomery, R.R.; et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest 2010, 90, 812–823. [Google Scholar]
- Chu, D.; Du, M.; Hu, X.; Wu, Q.; Shen, J. Paeoniflorin attenuates schistosomiasis japonica-associated liver fibrosis through inhibiting alternative activation of macrophages. Parasitology 2011, 138, 1259–1271. [Google Scholar]
- Gibbons, M.A.; Mackinnon, A.C.; Ramachandran, P.; Dhaliwal, K.; Duffin, R.; Phythian-Adams, A.T.; van Rooijen, N.; Haslett, C.; Howie, S.E.; Simpson, A.J. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med 2011, 184, 569–581. [Google Scholar]
- Ahmad, S.; North, B.V.; Qureshi, A.; Malik, A.; Bhangal, G.; Tarzi, R.M.; Brown, E.A.; Tam, F.W. CCL18 in peritoneal dialysis patients and encapsulating peritoneal sclerosis. Eur. J. Clin. Invest 2010, 40, 1067–1073. [Google Scholar]
- Bellon, T.; Martinez, V.; Lucendo, B.; Del, P.G.; Castro, M.J.; Aroeira, L.S.; Rodriguez-Sanz, A.; Ossorio, M.; Sanchez-Villanueva, R.; Selgas, R.; et al. Alternative activation of macrophages in human peritoneum: Implications for peritoneal fibrosis. Nephrol. Dial. Transplant 2011, 26, 2995–3005. [Google Scholar]
- Lopez-Navarrete, G.; Ramos-Martinez, E.; Suarez-Alvarez, K.; Aguirre-Garcia, J.; Ledezma-Soto, Y.; Leon-Cabrera, S.; Gudino-Zayas, M.; Guzman, C.; Gutierrez-Reyes, G.; Hernandez-Ruiz, J.; et al. Th2-associated alternative Kupffer cell activation promotes liver fibrosis without inducing local inflammation. Int. J. Biol. Sci 2011, 7, 1273–1286. [Google Scholar]
- Van Rooijen, N. The liposome-mediated macrophage “suicide” technique. J. Immunol. Methods 1989, 124, 1–6. [Google Scholar]
- Van Rooijen, N.; Van Nieuwmegen, R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res 1984, 238, 355–358. [Google Scholar]
- Murray, L.A.; Chen, Q.; Kramer, M.S.; Hesson, D.P.; Argentieri, R.L.; Peng, X.; Gulati, M.; Homer, R.J.; Russell, T.; van Rooijen, N.; et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int. J. Biochem. Cell Biol 2011, 43, 154–162. [Google Scholar]
- Biewenga, J.; van der Ende, M.B.; Krist, L.F.; Borst, A.; Ghufron, M.; van Rooijen, N. Macrophage depletion in the rat after intraperitoneal administration of liposome-encapsulated clodronate: depletion kinetics and accelerated repopulation of peritoneal and omental macrophages by administration of Freund's adjuvant. Cell Tissue Res 1995, 280, 189–196. [Google Scholar]
- Ha, H.; Cha, M.K.; Choi, H.N.; Lee, H.B. Effects of peritoneal dialysis solutions on the secretion of growth factors and extracellular matrix proteins by human peritoneal mesothelial cells. Perit. Dial. Int 2002, 22, 171–177. [Google Scholar]
- Kushiyama, T.; Oda, T.; Yamada, M.; Higashi, K.; Yamamoto, K.; Oshima, N.; Sakurai, Y.; Miura, S.; Kumagai, H. Effects of liposome-encapsulated clodronate on chlorhexidine gluconate-induced peritoneal fibrosis in rats. Nephrol. Dial. Transplant 2011, 26, 3143–3154. [Google Scholar]
- Huang, C.; Ogawa, R. Fibroproliferative disorders and their mechanobiology. Connect Tissue Res 2012, 53, 187–196. [Google Scholar]
- Takagawa, S.; Lakos, G.; Mori, Y.; Yamamoto, T.; Nishioka, K.; Varga, J. Sustained activation of fibroblast transforming growth factor-beta/Smad signaling in a murine model of scleroderma. J. Invest. Dermatol 2003, 121, 41–50. [Google Scholar]
- Lv, Z.D.; Wang, H.B.; Li, F.N.; Wu, L.; Liu, C.; Nie, G.; Kong, B.; Qu, H.L.; Li, J.G. TGF-beta1 induces peritoneal fibrosis by activating the Smad2 pathway in mesothelial cells and promotes peritoneal carcinomatosis. Int. J. Mol. Med 2012, 29, 373–379. [Google Scholar]
- Hu, W.; Jiang, Z.; Zhang, Y.; Liu, Q.; Fan, J.; Luo, N.; Dong, X.; Yu, X. Characterization of infiltrating macrophages in high glucose-induced peritoneal fibrosis in rats. Mol. Med. Report 2012, 6, 93–99. [Google Scholar]
- Van Rooijen, N.; Sanders, A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 1994, 174, 83–93. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001, 29, e45. [Google Scholar]
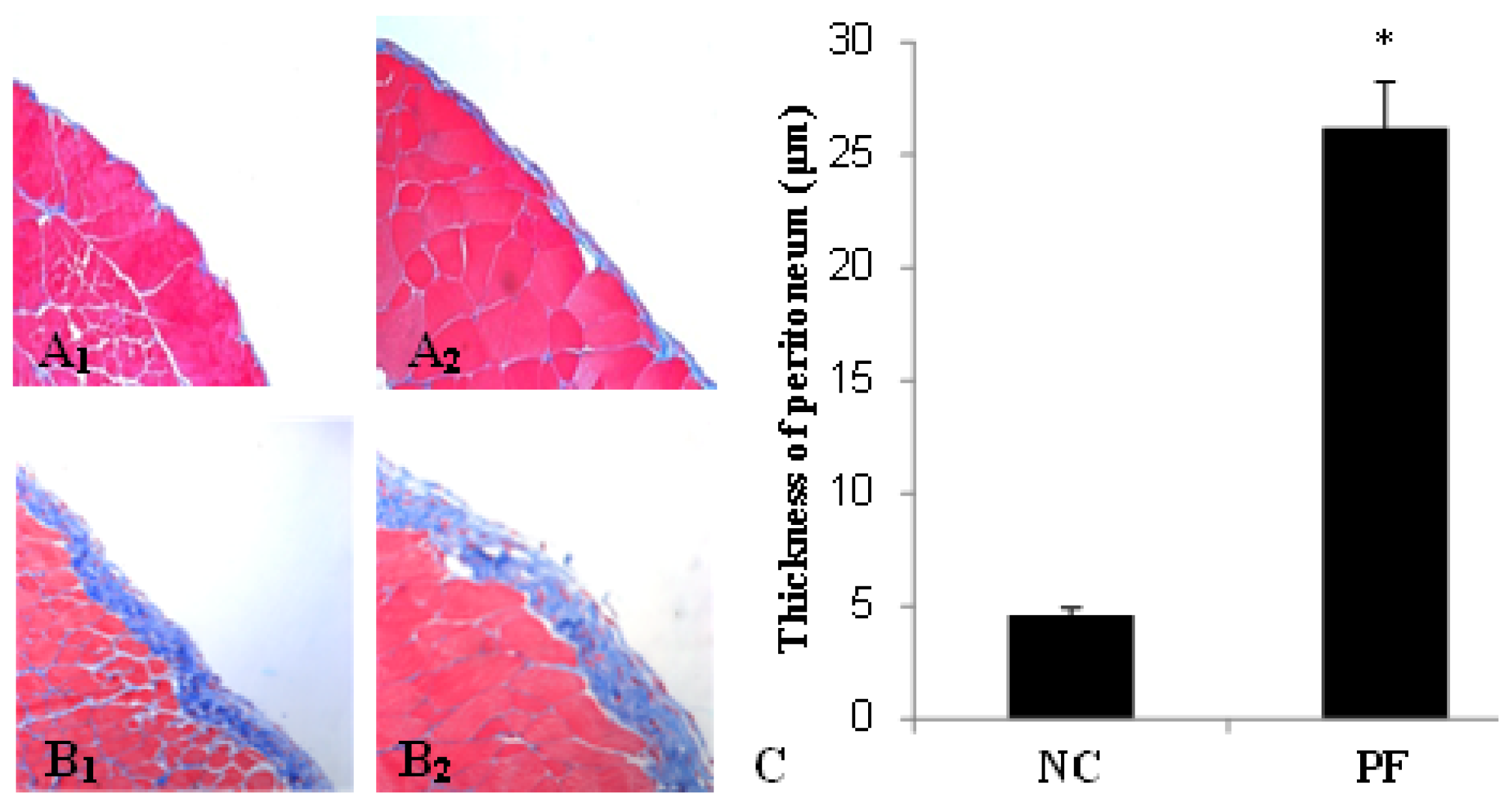


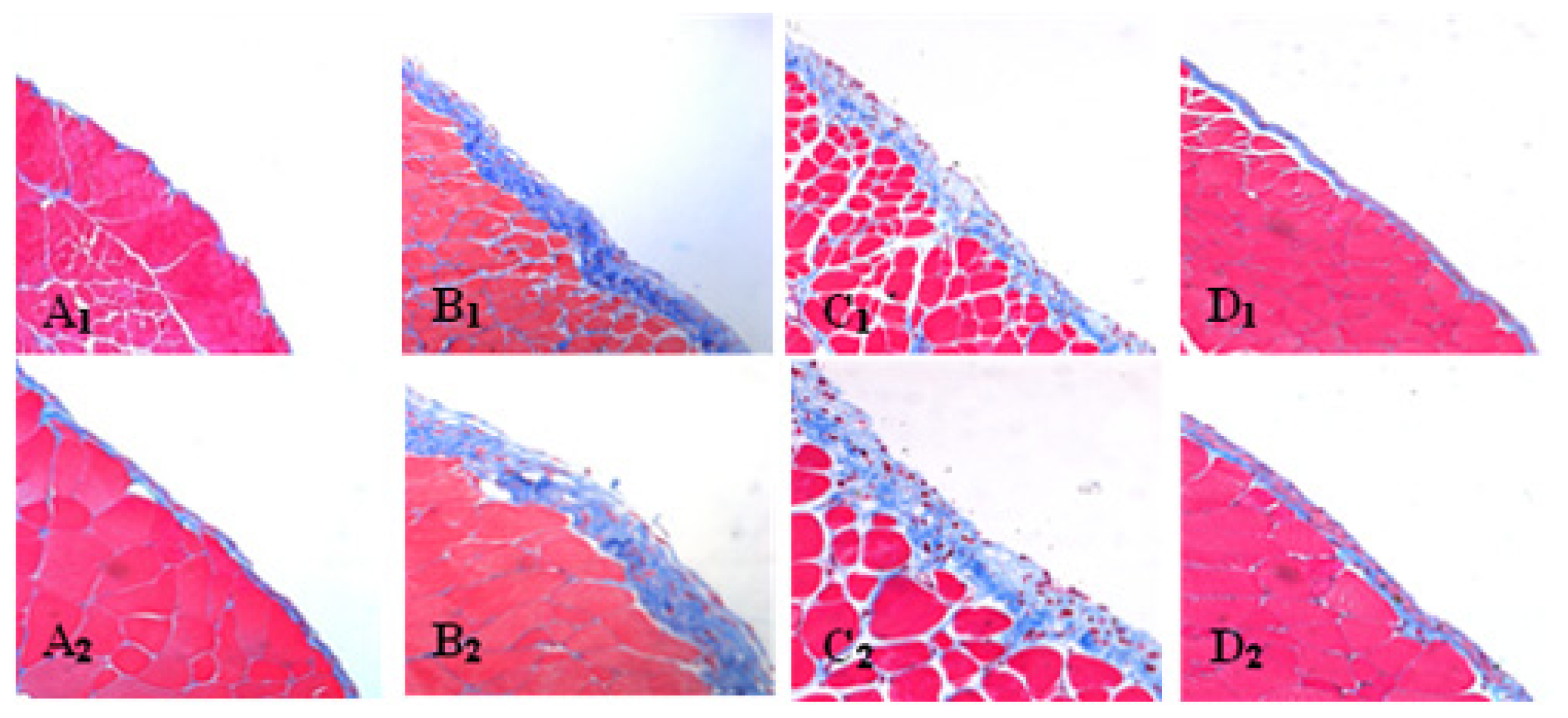
| Group | Max (μm) | Min (μm) | Mean ± SD |
|---|---|---|---|
| NC | 4.90 | 4.22 | 4.6 ± 0.34 |
| PF | 28.66 | 23.48 | 26.2 ± 2.03 # |
| LP | 33.73 | 20.96 | 27.7 ± 4.38 # |
| LC | 13.99 | 7.51 | 10.0 ± 2.36 |
| Gene | Primer sequence |
|---|---|
| β-actin | Forward: 5′-AGCCATGTACGTAGCCATCC-3′ Reverse: 5′-CTCTCAGCTGTGGTGGTG-3′ |
| Col-I | Forward: 5′-TTTGGAGAGAGCATGACCGA-3′ Reverse: 5′-TGCTGTAGGTGAAGCGACTGTT-3′ |
| Fibronectin | Forward: 5′-AATCACAGTAGTTGCGGCAGGAGA-3′ Reverse: 5′-TGTCATAGTCAATGCCAGGCTCCA-3′ |
| Arg-1 | Forward: 5′-AGAGCTGACAGCAACCCTGT-3′ Reverse: 5′-GGATCCAGAAGGTGATGGAA-3′ |
| Ym-1 | Forward: 5′-AATGATTCCTGCTCCTGT-3′ Reverse: 5′-ACTTTGATGGCCTCAACC-3′ |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, J.; Jiang, Z.-P.; Su, N.; Fan, J.-J.; Ruan, Y.-P.; Peng, W.-X.; Li, Y.-F.; Yu, X.-Q. The Role of Peritoneal Alternatively Activated Macrophages in the Process of Peritoneal Fibrosis Related to Peritoneal Dialysis. Int. J. Mol. Sci. 2013, 14, 10369-10382. https://doi.org/10.3390/ijms140510369
Wang J, Jiang Z-P, Su N, Fan J-J, Ruan Y-P, Peng W-X, Li Y-F, Yu X-Q. The Role of Peritoneal Alternatively Activated Macrophages in the Process of Peritoneal Fibrosis Related to Peritoneal Dialysis. International Journal of Molecular Sciences. 2013; 14(5):10369-10382. https://doi.org/10.3390/ijms140510369
Chicago/Turabian StyleWang, Jie, Zong-Pei Jiang, Ning Su, Jin-Jin Fan, Yi-Ping Ruan, Wen-Xing Peng, Ya-Fang Li, and Xue-Qing Yu. 2013. "The Role of Peritoneal Alternatively Activated Macrophages in the Process of Peritoneal Fibrosis Related to Peritoneal Dialysis" International Journal of Molecular Sciences 14, no. 5: 10369-10382. https://doi.org/10.3390/ijms140510369
APA StyleWang, J., Jiang, Z. -P., Su, N., Fan, J. -J., Ruan, Y. -P., Peng, W. -X., Li, Y. -F., & Yu, X. -Q. (2013). The Role of Peritoneal Alternatively Activated Macrophages in the Process of Peritoneal Fibrosis Related to Peritoneal Dialysis. International Journal of Molecular Sciences, 14(5), 10369-10382. https://doi.org/10.3390/ijms140510369




