Surgical Resection of Brain Metastases—Impact on Neurological Outcome
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Conflict of Interest
References
- Alexandru, D.; Bota, D.A.; Linskey, M.E. Epidemiology of central nervous system metastases. Prog. Neurol. Surg 2012, 25, 13–29. [Google Scholar]
- Tabouret, E.; Chinot, O.; Metellus, P.; Tallet, A.; Viens, P.; Goncalves, A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res 2012, 32, 4655–4662. [Google Scholar]
- Barajas, R.F., Jr; Cha, S. Imaging diagnosis of brain metastasis. Prog. Neurol. Surg 2012, 25, 55–73. [Google Scholar]
- Burstein, H.J.; Lieberman, G.; Slamon, D.J.; Winer, E.P.; Klein, P. Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann. Oncol 2005, 16, 1772–1777. [Google Scholar]
- Azim, H.A.; Azim, H.A., Jr. Systemic treatment of brain metastases in HER2-positive breast cancer: Current status and future directions. Future Oncol 2012, 8, 135–144. [Google Scholar]
- Mehta, A.I.; Brufsky, A.M.; Sampson, J.H. Therapeutic approaches for HER2-positive brain metastases: Circumventing the blood-brain barrier. Cancer Treat. Rev 2012, 39, 261–269. [Google Scholar]
- Scoccianti, S.; Ricardi, U. Treatment of brain metastases: review of phase III randomized controlled trials. Radiother. Oncol 2012, 102, 168–179. [Google Scholar]
- Chang, E.L.; Wefel, J.S.; Maor, M.H.; Hassenbusch, S.J., III; Mahajan, A.; Lang, F.F.; Woo, S.Y.; Mathews, L.A.; Allen, P.K.; Shiu, A.S.; et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery 2007, 60, 277–283, discussion 283–284. [Google Scholar]
- Al-Shamy, G.; Sawaya, R. Management of brain metastases: The indispensable role of surgery. J. Neurooncol 2009, 92, 275–282. [Google Scholar]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med 1990, 322, 494–500. [Google Scholar]
- Vecht, C.J.; Haaxma-Reiche, H.; Noordijk, E.M.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann. Neurol 1993, 33, 583–590. [Google Scholar]
- Narita, Y.; Shibui, S. Strategy of surgery and radiation therapy for brain metastases. Int. J. Clin. Oncol 2009, 14, 275–280. [Google Scholar]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys 1997, 37, 745–751. [Google Scholar]
- Tendulkar, R.D.; Liu, S.W.; Barnett, G.H.; Vogelbaum, M.A.; Toms, S.A.; Jin, T.; Suh, J.H. RPA classification has prognostic significance for surgically resected single brain metastasis. Int. J. Radiat. Oncol. Biol. Phys 2006, 66, 810–817. [Google Scholar]
- Caissie, A.; Nguyen, J.; Chen, E.; Zhang, L.; Sahgal, A.; Clemons, M.; Kerba, M.; Arnalot, P.F.; Danjoux, C.; Tsao, M.; et al. Quality of life in patients with brain metastases using the EORTC QLQ-BN20+2 and QLQ-C15-PAL. Int. J. Radiat. Oncol. Biol. Phys 2011, 83, 1238–1245. [Google Scholar]
- Cox, D.R. Regression models and life tables. J. R. Stat. Soc 1972, 34, 197–199. [Google Scholar]
- Sen, M.; Demiral, A.S.; Cetingoz, R.; Alanyali, H.; Akman, F.; Senturk, D.; Kinay, M. Prognostic factors in lung cancer with brain metastasis. Radiother. Oncol 1998, 46, 33–38. [Google Scholar]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin 2010, 60, 277–300. [Google Scholar]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar]
- Mintz, A.H.; Kestle, J.; Rathbone, M.P.; Gaspar, L.; Hugenholtz, H.; Fisher, B.; Duncan, G.; Skingley, P.; Foster, G.; Levine, M. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996, 78, 1470–1476. [Google Scholar]
- Mut, M. Surgical treatment of brain metastasis: A review. Clin. Neurol. Neurosurg 2012, 114, 1–8. [Google Scholar]
- Kalkanis, S.N.; Kondziolka, D.; Gaspar, L.E.; Burri, S.H.; Asher, A.L.; Cobbs, C.S.; Ammirati, M.; Robinson, P.D.; Andrews, D.W.; Loeffler, J.S.; et al. The role of surgical resection in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neurooncol 2010, 96, 33–43. [Google Scholar]
- Ranasinghe, M.G.; Sheehan, J.M. Surgical management of brain metastases. Neurosurg. Focus 2007, 22, E2. [Google Scholar]
- Zhang, X.; Zhang, W.; Cao, W.D.; Cheng, G.; Liu, B.; Cheng, J. A review of current management of brain metastases. Ann. Surg. Oncol 2012, 19, 1043–1050. [Google Scholar]
- Colen, R.R.; Kekhia, H.; Jolesz, F.A. Multimodality intraoperative MRI for brain tumor surgery. Expert Rev. Neurother 2010, 10, 1545–1558. [Google Scholar]
- Willems, P.W.; van der Sprenkel, J.W.; Tulleken, C.A.; Viergever, M.A.; Taphoorn, M.J. Neuronavigation and surgery of intracerebral tumours. J. Neurol 2006, 253, 1123–1136. [Google Scholar]
- Tan, T.C.; Mc, L.B.P. Image-guided craniotomy for cerebral metastases: Techniques and outcomes. Neurosurgery 2003, 53, 82–89, , discussion 89–90.. [Google Scholar]
- Chacko, A.G.; Thomas, S.G.; Babu, K.S.; Daniel, R.T.; Chacko, G.; Prabhu, K.; Cherian, V.; Korula, G. Awake craniotomy and electrophysiological mapping for eloquent area tumours. Clin. Neurol. Neurosurg 2012, 115, 329–334. [Google Scholar]
- Bindal, A.K.; Bindal, R.K.; Hess, K.R.; Shiu, A.; Hassenbusch, S.J.; Shi, W.M.; Sawaya, R. Surgery versus radiosurgery in the treatment of brain metastasis. J. Neurosurg 1996, 84, 748–754. [Google Scholar]
- Al-Zabin, M.; Ullrich, W.O.; Brawanski, A.; Proescholdt, M.A. Recurrent brain metastases from lung cancer: The impact of reoperation. Acta Neurochir. (Wien.) 2010, 152, 1887–1892. [Google Scholar]
- Sawaya, R.; Hammoud, M.; Schoppa, D.; Hess, K.R.; Wu, S.Z.; Shi, W.M.; Wildrick, D.M. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 1998, 42, 1044–1055, , discussion 1055–1056.. [Google Scholar]
- Mintz, A.; Perry, J.; Spithoff, K.; Chambers, A.; Laperriere, N. Management of single brain metastasis: A practice guideline. Curr. Oncol 2007, 14, 131–143. [Google Scholar]
- O’Neill, B.P.; Iturria, N.J.; Link, M.J.; Pollock, B.E.; Ballman, K.V.; O’Fallon, J.R. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int. J. Radiat. Oncol. Biol. Phys 2003, 55, 1169–1176. [Google Scholar]
- Schoggl, A.; Kitz, K.; Reddy, M.; Wolfsberger, S.; Schneider, B.; Dieckmann, K.; Ungersbock, K. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir. (Wien.) 2000, 142, 621–626. [Google Scholar]
- Redmond, A.J.; Diluna, M.L.; Hebert, R.; Moliterno, J.A.; Desai, R.; Knisely, J.P.; Chiang, V.L. Gamma Knife surgery for the treatment of melanoma metastases: The effect of intratumoral hemorrhage on survival. J. Neurosurg 2008, 109, 99–105. [Google Scholar]
- Elliott, R.E.; Rush, S.; Morsi, A.; Mehta, N.; Spriet, J.; Narayana, A.; Donahue, B.; Parker, E.C.; Golfinos, J.G. Neurological complications and symptom resolution following Gamma Knife surgery for brain metastases 2 cm or smaller in relation to eloquent cortices. J. Neurosurg 2010, 113, 53–64. [Google Scholar]
- Williams, B.J.; Suki, D.; Fox, B.D.; Pelloski, C.E.; Maldaun, M.V.; Sawaya, R.E.; Lang, F.F.; Rao, G. Stereotactic radiosurgery for metastatic brain tumors: A comprehensive review of complications. J. Neurosurg 2009, 111, 439–448. [Google Scholar]
- Siu, T.L.; Jeffree, R.L.; Fuller, J.W. Current strategies in the surgical management of cerebral metastases: An evidence-based review. J. Clin. Neurosci 2011, 18, 1429–1434. [Google Scholar]
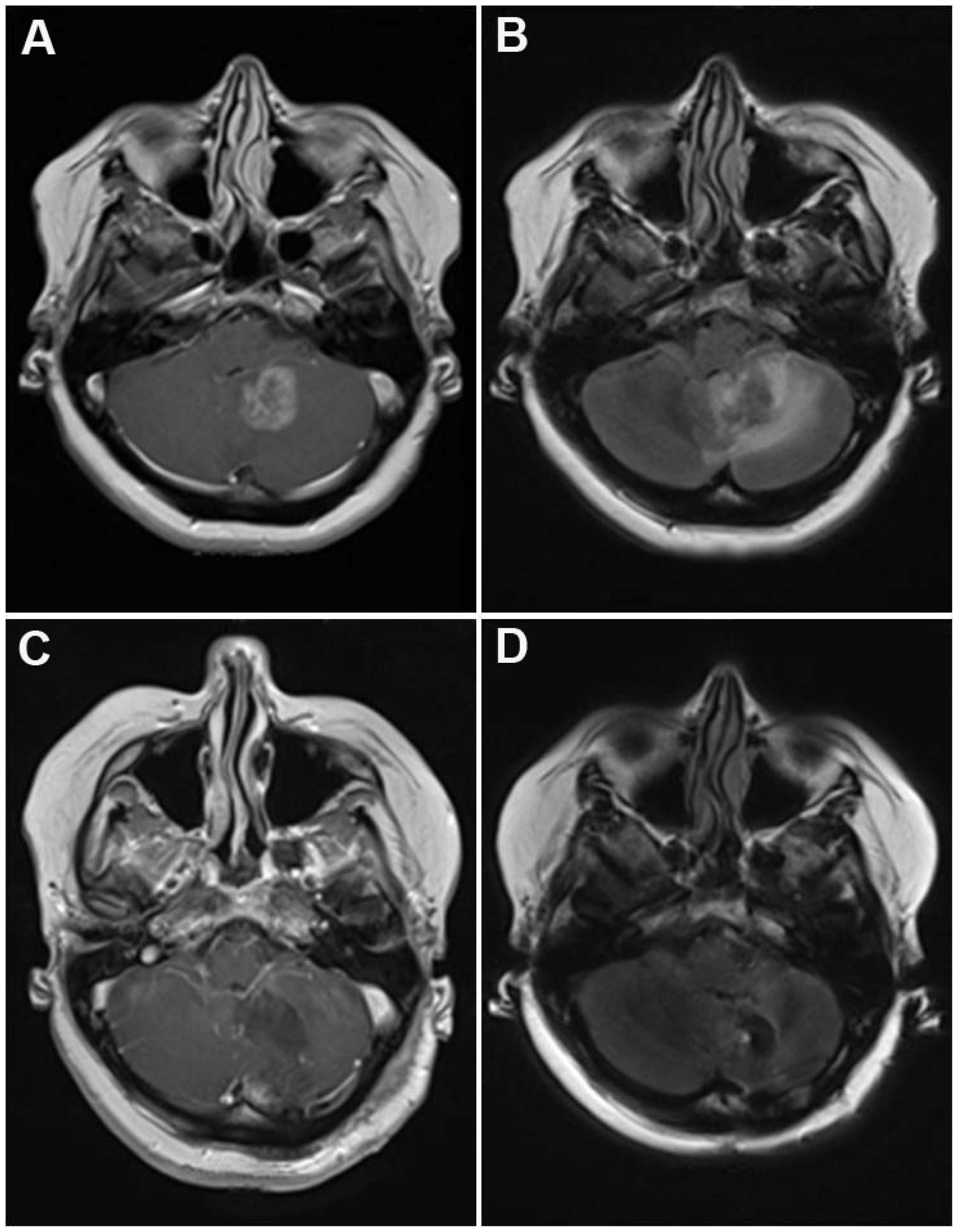
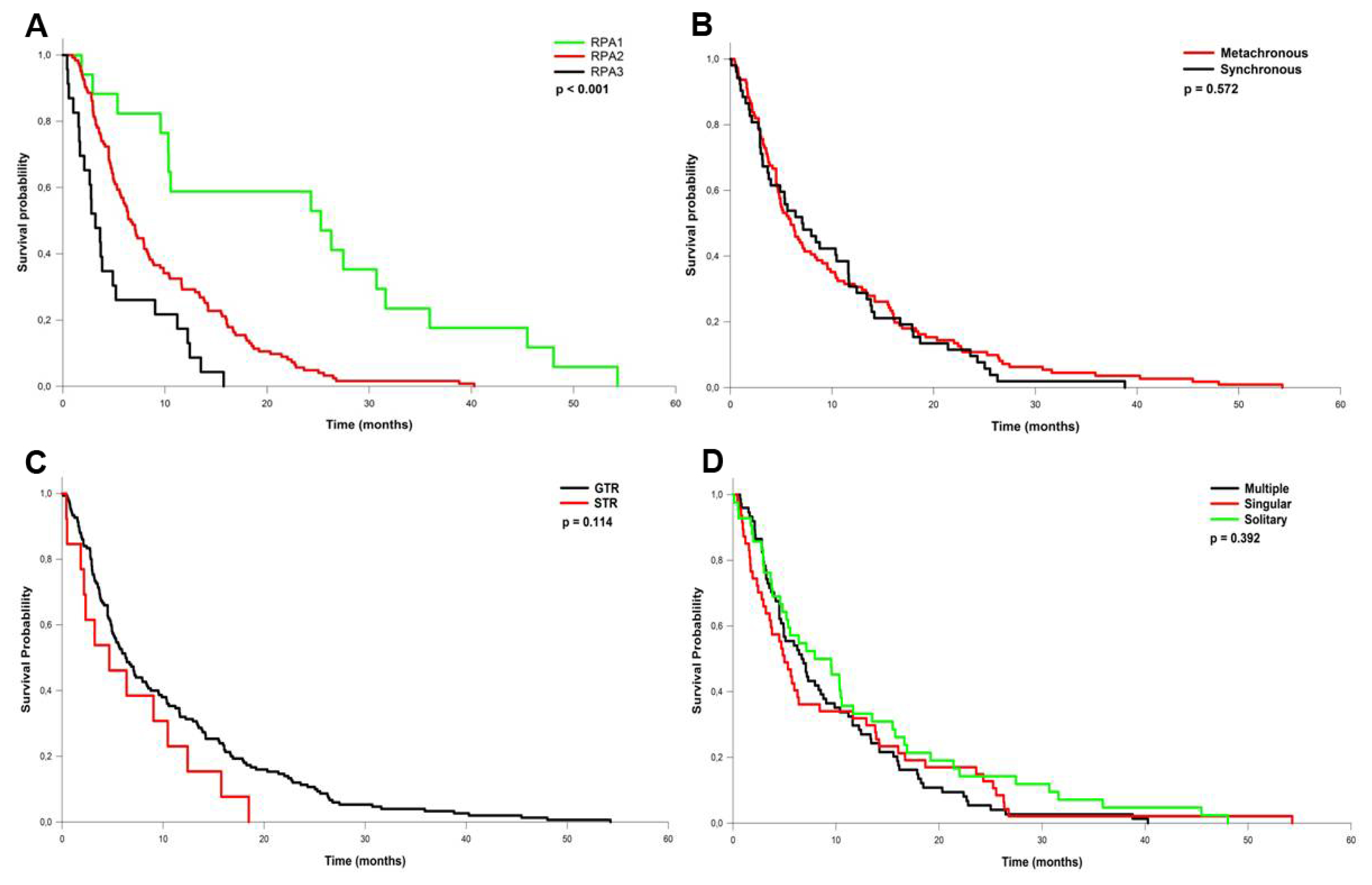
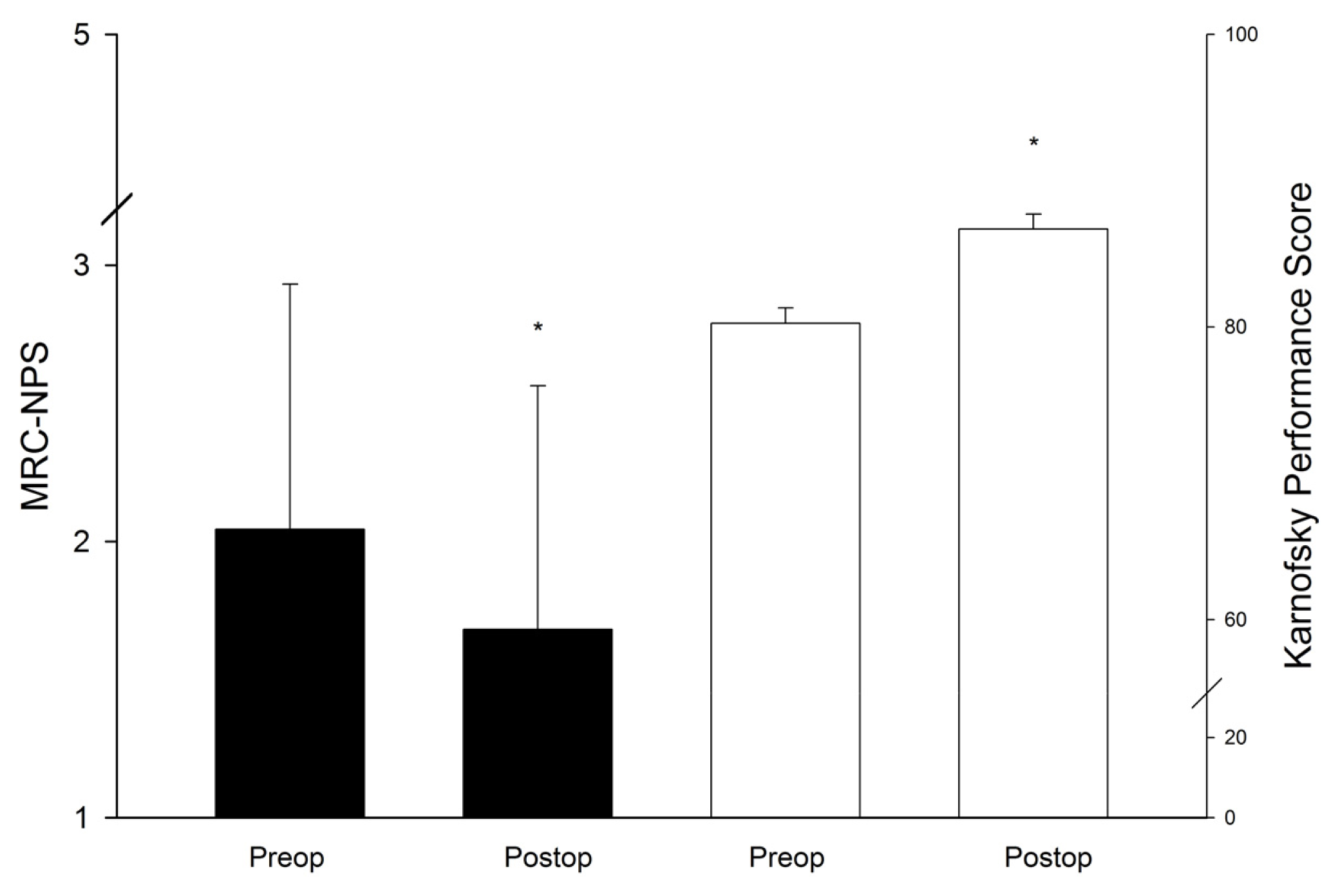
| Variable | Number | % |
|---|---|---|
| Age (years) | ||
| Mean | 61.6 | |
| Range | 23.4–83.9 | |
| Gender | ||
| Male | 122 | 59.2 |
| Female | 84 | 40.8 |
| Primary tumor | ||
| Lung cancer | 70 | 34.0 |
| Melanoma | 30 | 14.5 |
| Breast cancer | 28 | 13.6 |
| Colon cancer | 20 | 9.7 |
| Renal cancer | 16 | 7.8 |
| CUP | 9 | 4.4 |
| Urothel cancer | 7 | 3.4 |
| Prostate | 4 | 1.9 |
| Other | 22 | 10.7 |
| Systemic disease | ||
| Controlled | 99 | 48.1 |
| Active | 107 | 51.9 |
| Time of brain metastases | ||
| Synchronous | 64 | 31.1 |
| Metachronous | 142 | 68.9 |
| Status of metastasis | ||
| Solitary | 61 | 29.7 |
| Singular | 60 | 29.1 |
| Multiple | 85 | 41.2 |
| Grade | Performance |
|---|---|
| 1 | No neurological deficit |
| 2 | Some neurological deficit but function adequate for useful work |
| 3 | Neurological deficit causing moderate functional impairment e.g., ability to move limbs only with difficulty, moderate dysphasia, moderate paresis, some visual disturbance |
| 4 | Neurological deficit causing major functional impairment e.g., inability to use limbs, gross speech or visual disturbances |
| 5 | No useful function-inability to make conscious responses |
| Surgical morbidity | Patients | % |
|---|---|---|
| CSF leakage | 9 | 4.4 |
| Hemorrhage | 6 | 2.9 |
| Wound infection | 3 | 1.5 |
| Stroke | 2 | 1.0 |
| New seizure | 1 | 0.5 |
| n | 21 | 10.3 |
| Neurological morbidity | ||
| New neurological deficit | 4 | 1.9 |
| Worsening of existing deficit | 9 | 4.4 |
| n | 13 | 6.3 |
| Total morbidity | 34 | 16.6 |
| Median survival (months) | 1-year survival rate (%) | 2-year survival rate (%) | |
|---|---|---|---|
| all | 6.3 | 24.6 | 8.2 |
| RPA 1 | 25.2 | 43.5 | 39.1 |
| RPA 2 | 6.7 | 22.4 | 3.7 |
| RPA 3 | 3.2 | 21.7 | 4.3 |
| Parameter | Hazard ratio | 95% CI Low | High | p |
|---|---|---|---|---|
| Age | 0.02 | 0.993 | 1.021 | 0.882 |
| Tumor size | 2.26 | 0.958 | 1.282 | 0.132 |
| Primary tumor | 0.06 | 0.926 | 1.118 | 0.801 |
| Metachronous/synchronous | 0.56 | 0.856 | 1.987 | 0.454 |
| RPA class | 13.70 | 1.262 | 2.617 | 0.001 |
| Solitary/singular/multiple | 1.53 | 0.862 | 1.308 | 0.215 |
| Time interval to metastasis | 15.50 | 0.982 | 1.001 | 0.001 |
| Parameter | Pre-OP n (%) | Stable n (% affected) | Resolved n (% affected) | Improved n (% affected) | Worsened n (% affected) | p |
|---|---|---|---|---|---|---|
| Increased ICP | 84 (40.8%) | 1 (1.2%) | 82 (97.6%) | 1 (1.2%) | 0 | 0.001 |
| Hemiparesis | 21 (10.2%) | 12 (57.1%) | 3 (14.3%) | 6 (28.6%) | 0 | 0.014 |
| Aphasia | 25 (12.1%) | 11 (44.0%) | 4 (16.0%) | 3 (12.0%) | 7 (28.0%) | 0.334 |
| Visual field defect | 21 (10.2%) | 18 (85.7%) | 1 (4.8%) | 0 | 2 (9.5%) | 0.894 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schödel, P.; Schebesch, K.-M.; Brawanski, A.; Proescholdt, M.A. Surgical Resection of Brain Metastases—Impact on Neurological Outcome. Int. J. Mol. Sci. 2013, 14, 8708-8718. https://doi.org/10.3390/ijms14058708
Schödel P, Schebesch K-M, Brawanski A, Proescholdt MA. Surgical Resection of Brain Metastases—Impact on Neurological Outcome. International Journal of Molecular Sciences. 2013; 14(5):8708-8718. https://doi.org/10.3390/ijms14058708
Chicago/Turabian StyleSchödel, Petra, Karl-Michael Schebesch, Alexander Brawanski, and Martin Andreas Proescholdt. 2013. "Surgical Resection of Brain Metastases—Impact on Neurological Outcome" International Journal of Molecular Sciences 14, no. 5: 8708-8718. https://doi.org/10.3390/ijms14058708




