The Interaction of CuS and Halothiobacillus HT1 Biofilm in Microscale Using Synchrotron Radiation-Based Techniques
Abstract
:1. Introduction
2. Results
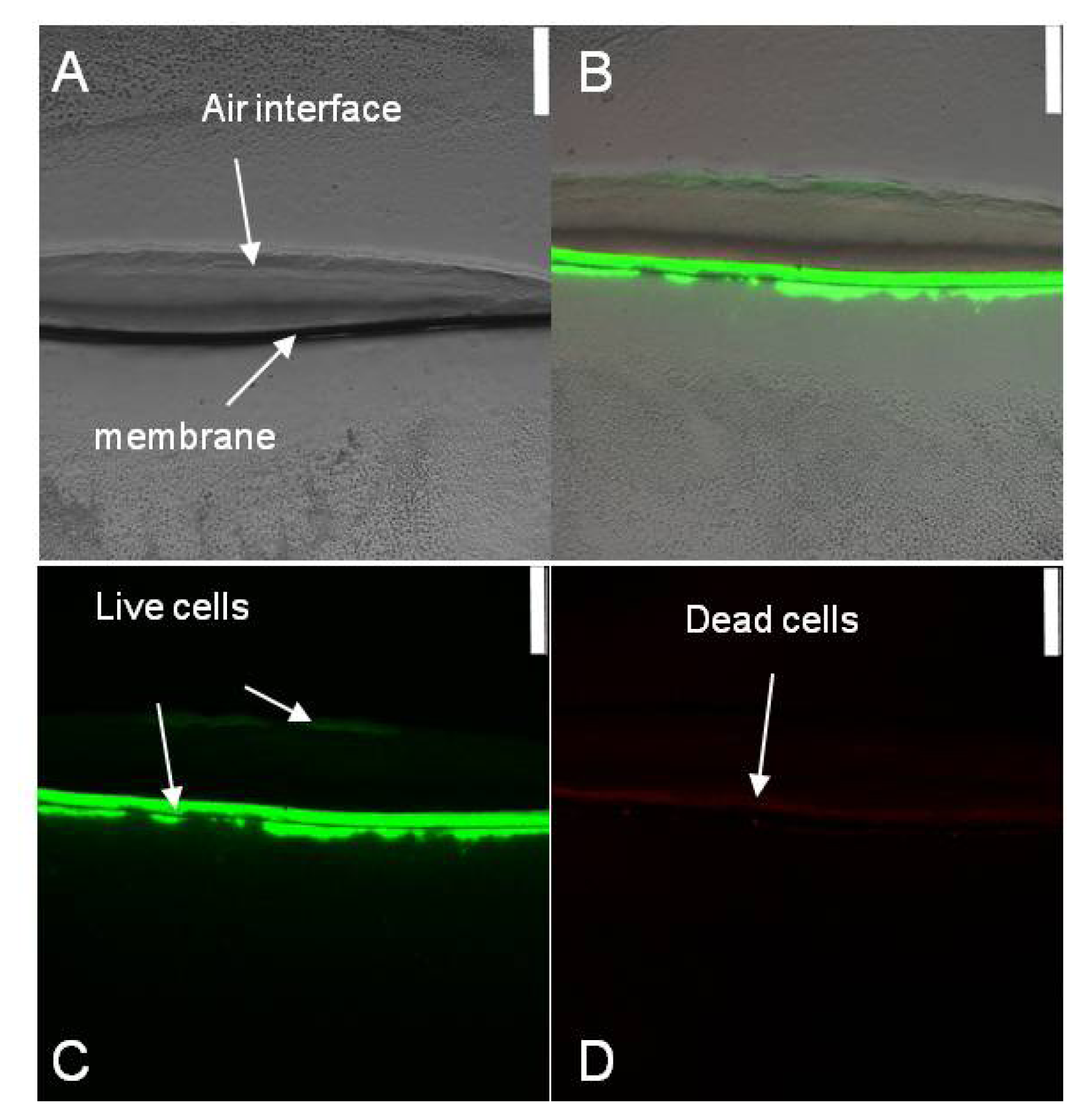
2.1. Spatial Distribution of Live and Dead Halothiobacillus HT1 Cells in the Biofilms
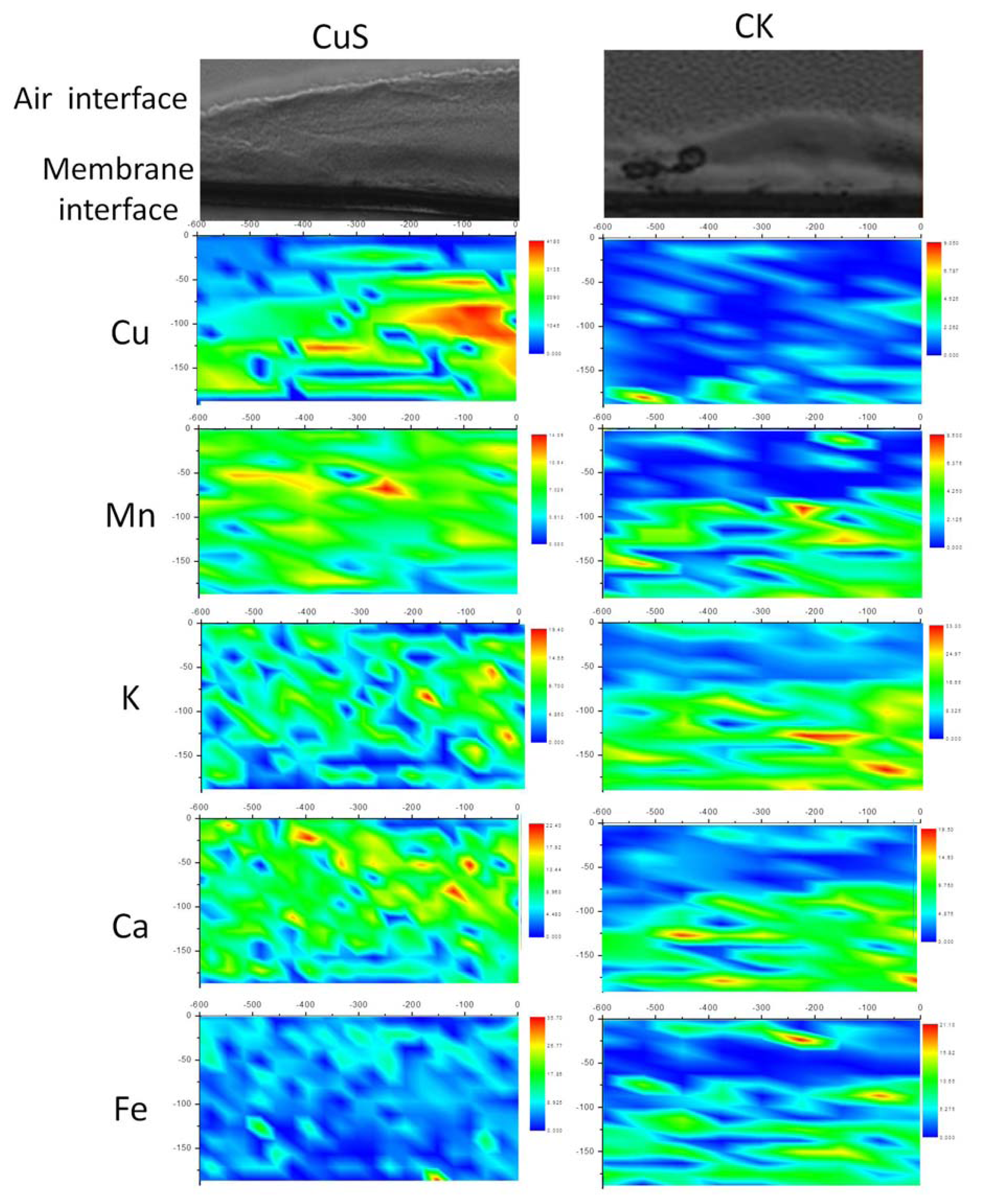
2.2. Spatial Distributions of Cu in Biofilm and Cu Speciation
2.3. Sulfur Speciation in the Biofilms
3. Discussion
3.1. The Relationship of Cu Distribution and Live/Dead Cells in Biofilms
3.2. Effects of Halothiobacillus HT1 Biofilms on CuS Transformation
4. Experimental Section
4.1. Biofilms Cultivation
4.2. Live/Dead Cells Distribution Using CLSM Image
4.3. Distribution of Cu in the Biofilm by Using Synchrotron-Based X-Ray Fluorescence
4.4. Copper Speciation Analysis Using Cu K-Edge Micro-XANES
4.5. Sulfur Speciation Analysis Using Sulfur K-Edge XANES
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Dynes, J.J.; Tyliszczak, T.; Araki, T.; Lawrence, J.R.; Swerhone, G.D.W.; Leppard, G.G.; Hitchcock, A.P. Speciation and quantitative mapping of metal species in microbial biofilms using scanning transmission X-ray Microscopy. Environ. Sci. Technol 2006, 40, 1556–1565. [Google Scholar]
- Geoffrey, M.G. Microbial influence on metal mobility and application for bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar]
- Ito, T.; Sugita, K.; Okabe, S. Isolation, characterization, and in situ detection of a novel chemolithoautotrophic sulfur-oxidizing bacterium in wastewater biofilms growing under microaerophilic conditions. Appl. Environ. Microbiol 2004, 70, 3122–3129. [Google Scholar]
- Ma, S.; Banfield, J.F. Micron-scale Fe2+/Fe3+, intermediate sulfur species and O2 gradients across the biofilm-solution-sediment interface control biofilm organization. Geochim. Cosmochim. Ac 2011, 75, 3568–3580. [Google Scholar]
- Edwards, K.J.; Bond, P.L.; Druschel, G.K.; McGuire, M.M.; Hamers, R.J.; Banfield, J.F. Geochemical and biological aspects of sulfide mineral dissolution: Lessons from Iron Mountain, California. Chem. Geol 2000, 169, 383–397. [Google Scholar]
- Templeton, A.; Knowles, E. Microbial transformations of minerals and metals: Recent advances in geomicrobiology derived from synchrotron-based X-ray spectroscopy and X-ray microscopy. Ann. Rev. Earth. Pl. Sc 2009, 37, 367–391. [Google Scholar]
- Zachara, J.M.; Fredrickson, J.K.; Smith, S.C.; Gassman, P.L. Solubilization of Fe(III) oxide-bound trace metals by a dissimilatory Fe(III) reducing bacterium. Geochim. Cosmochim. Ac 2001, 65, 75–93. [Google Scholar]
- Templeton, A.S.; Trainor, T.P.; Traina, S.J.; Spormann, A.M.; Brown, G.E. Pb(II) distributions at biofilm-metal oxide interfaces. Proc. Natl. Acad. Sci. USA 2001, 98, 11897–11902. [Google Scholar]
- Drenkard, E.; Ausubel, F.M. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 2002, 416, 740–743. [Google Scholar]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol 2005, 13, 34–40. [Google Scholar]
- Sandt, C.; Smith-Palmer, T.; Pink, J.; Brennan, L.; Pink, D. Confocal Raman microspectroscopy as a tool for studying the chemical heterogeneities of biofilms in situ. J. Appl. Microbiol 2007, 103, 1808–1820. [Google Scholar]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol 2007, 5, 928–938. [Google Scholar]
- Harrison, J.J.; Ceri, H.; Stremick, C.A.; Turner, R.J. Biofilm susceptibility to metal toxicity. Environ. Microbiol 2004, 6, 1220–1227. [Google Scholar]
- Butcher, B.G.; Deane, S.M.; Rawlings, D.E. The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl. Environ. Microbiol 2000, 66, 1826–1833. [Google Scholar]
- Shi, J.Y.; Lin, H.R.; Yuan, X.F.; Zhao, Y.D. Isolation and characterization of a novel sulfur-oxidizing chemolithoautotroph Halothiobacillus from Pb polluted paddy soil. Afr. J. Biotechnol 2011, 10, 4121–4126. [Google Scholar]
- Ortega, R. Chemical elements distribution in cells. Nucl. Instr. Meth. Phys. Res 2005, 231, 218–223. [Google Scholar]
- Rani, S.A.; Pitts, B.; Beyenal, H.; Veluchamy, R.A.; Lewandowski, Z.; Davison, W.M.; Buckingham-Meyer, K.; Stewart, P.S. Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states. J. Bacteriol 2007, 189, 4223–4233. [Google Scholar]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol 2004, 2, 95–108. [Google Scholar]
- Battin, T.J.; Sloan, W.T.; Kjelleberg, S.; Daims, H.; Head, I.M.; Curtis, T.P.; Eberl, L. Microbial landscapes: New paths to biofilm research. Appl. Environ. Microbiol 2007, 5, 76–81. [Google Scholar]
- Sauer, K.; Camper, A.K.; Ehrlich, G.D.; Costerton, J.W.; Davies, D.G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol 2002, 184, 1140–1154. [Google Scholar]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol 2008, 6, 199–210. [Google Scholar]
- Chen, G.C.; Chen, X.C.; Yang, Y.Q.; Hay, A.G.; Yu, X.H.; Chen, Y.X. Sorption and distribution of copper in unsaturated Pseudomonas putida CZ1 biofilms as determined by X-ray fluorescence microscopy. Appl. Environ. Microbiol 2011, 77, 4719–4727. [Google Scholar]
- Fahrni, C.J. Biological applications of X-ray fluorescence microscopy: Exploring the subcellular topography and speciation of transition metals. Curr. Opin. Chem. Biol 2007, 11, 121–127. [Google Scholar]
- Hu, Z.Q.; Hidalgo, G.; Houston, P.L.; Hay, A.G.; Shuler, M.L.; Abruna, H.D.; Ghiorse, W.C.; Lion, L.W. Determination of spatial distributions of zinc and active biomass in microbial biofilms by two-photon laser scanning microscopy. Appl. Environ. Microbiol 2005, 71, 4014–4021. [Google Scholar]
- Silver, S.; Phung, L.T. Bacterial heavy metal resistance: New surprises. Ann. Rev. Microbiol 1996, 50, 753–789. [Google Scholar]
- Alvarez, S.; Jerez, C.A. Copper ions stimulate polyphosphate degradation and phosphate efflux in Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol 2004, 70, 5177–5182. [Google Scholar]
- Kornberg, A.; Rao, N.N.; Ault-Richer, D. Inorganic polyphosphate: A molecule of many functions. Ann. Rev. Biochem 1999, 68, 89–125. [Google Scholar]
- Remonsellez, F.; Orell, A.; Jerez, C.A. Copper tolerance of the thermoacidophilic archaeon Sulfolobus metallicus: Possible role of polyphosphate metabolism. Microbiology 2006, 152, 59–66. [Google Scholar]
- Kim, B.E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol 2008, 4, 176–185. [Google Scholar]
- Quaranta, D.; McCarty, R.; Bandarian, V.; Rensing, C. The copper-inducible cin operon encodes an unusual methionine-rich azurin-like protein and a pre-Q0 reductase in Pseudomonas putida KT2440. J. Bacteriol 2007, 189, 5361–5371. [Google Scholar]
- PyMca. Available online: http://pymca.sourceforge.net (accessed on 15 July 2011).
- Shi, J.Y.; Wu, B.; Yuan, X.F.; Cao, Y.Y.; Chen, X.C.; Chen, Y.X.; Hu, T.D. An X-ray absorption spectroscopy investigation of speciation and biotransformation of copper in Elsholtzia splendens. Plant Soil 2008, 302, 163–174. [Google Scholar]
- Lin, H.R.; Shi, J.Y.; Chen, X.C.; Yang, J.J.; Chen, Y.X.; Zhao, Y.D.; Hu, T.D. Effects of lead upon the actions of sulfate-reducing bacteria in the rice rhizosphere. Soil Biol. Biochem 2010, 42, 1038–1044. [Google Scholar]
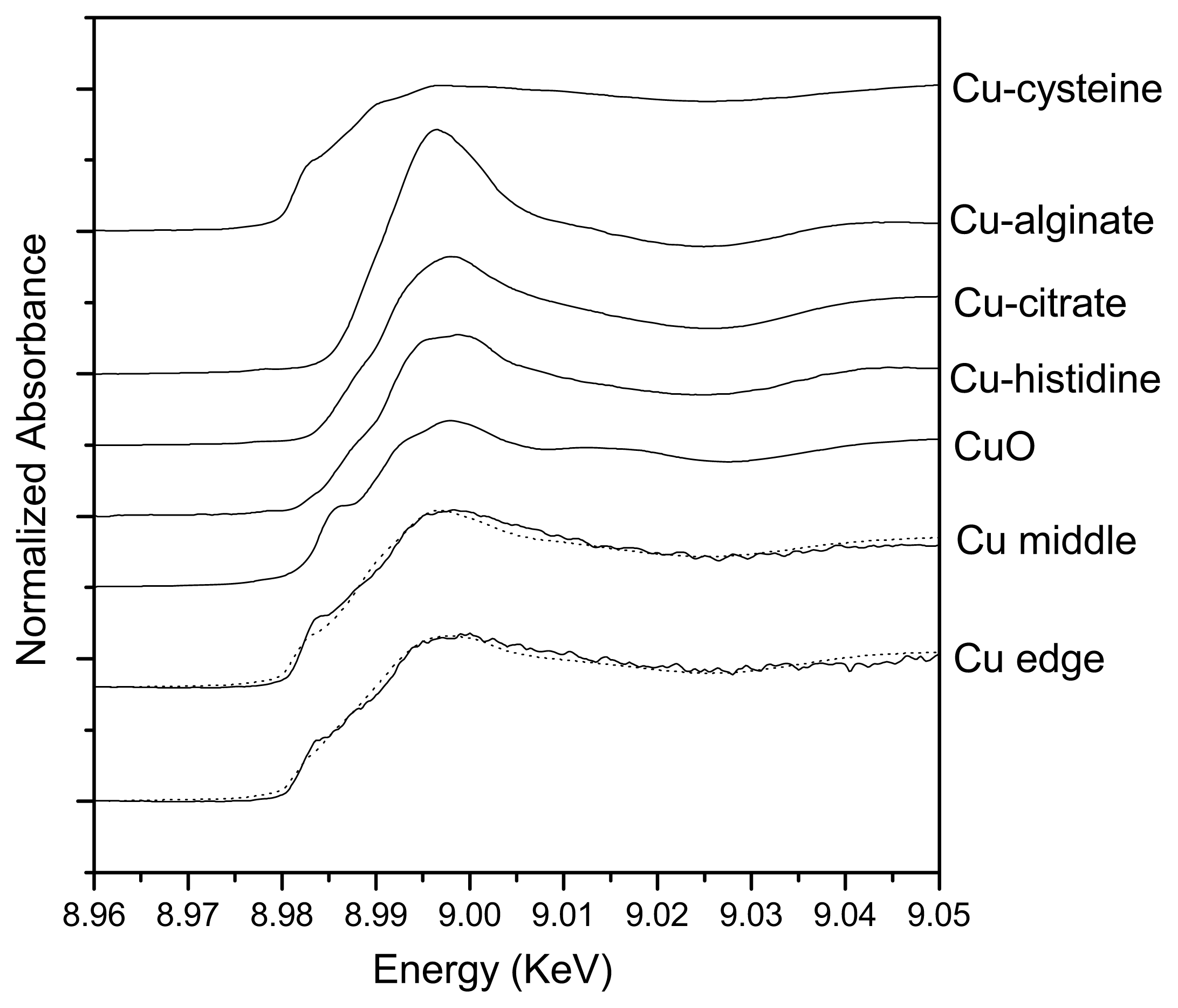
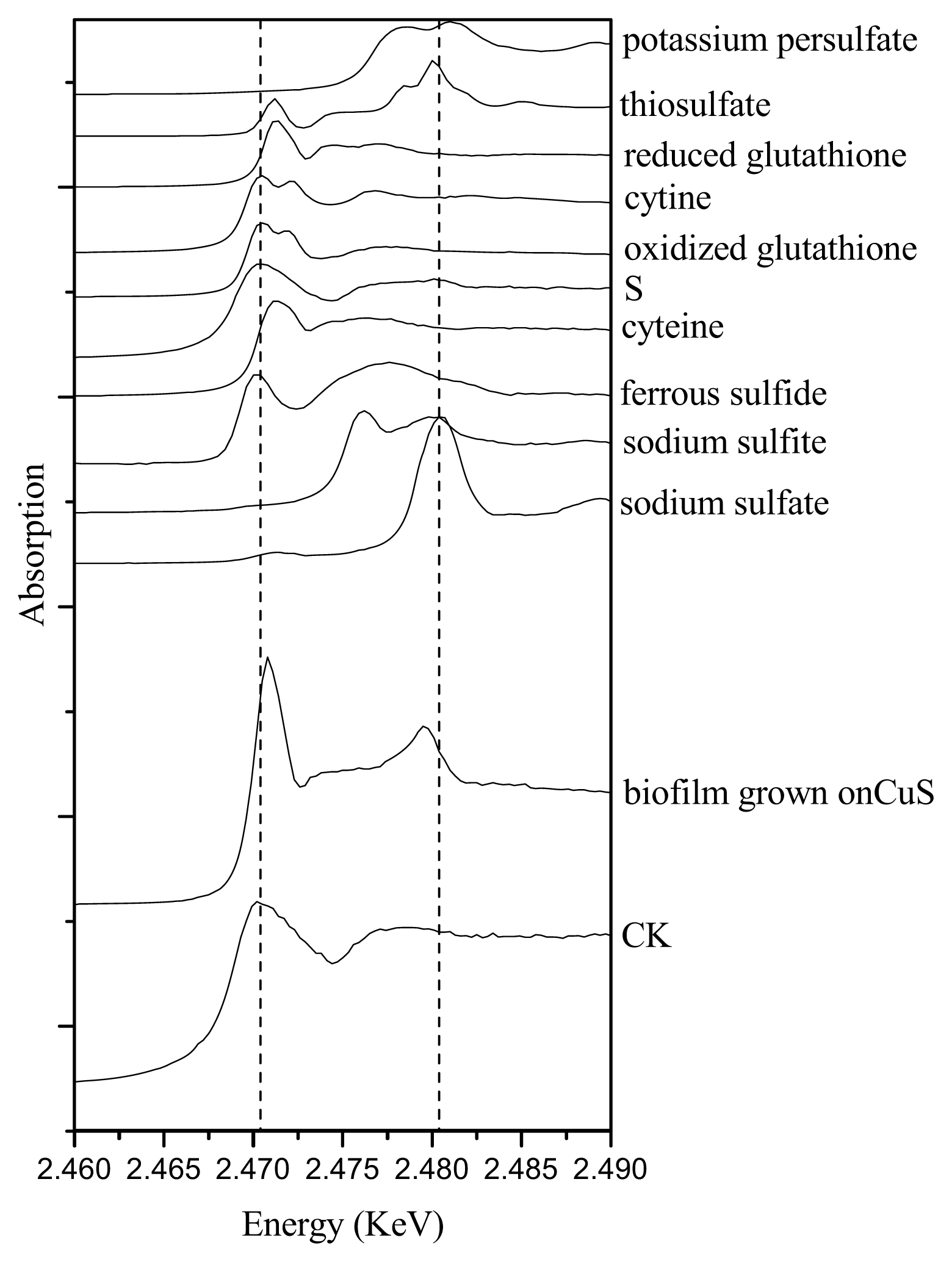
| Samples | Cu-cysteine (%) | Cu-alginate (%) | Cu-citrate (%) | Cu-histidine (%) | CuO (%) | Rss |
|---|---|---|---|---|---|---|
| Center | 67.8 | 21.4 | 10.8 | - | - | 0.51 |
| Edge | 48.4 | 2.9 | 7.1 | 22.3 | 19.3 | 0.51 |
| Treatments | Sulfur | Cysteine | Sulfite | Sulfonate |
|---|---|---|---|---|
| CK | 100 | 0 | 0 | 0 |
| CuS | 0 | 77.3 | 3.8 | 18.9 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, H.; Chen, G.; Zhu, S.; Chen, Y.; Chen, D.; Xu, W.; Yu, X.; Shi, J. The Interaction of CuS and Halothiobacillus HT1 Biofilm in Microscale Using Synchrotron Radiation-Based Techniques. Int. J. Mol. Sci. 2013, 14, 11113-11124. https://doi.org/10.3390/ijms140611113
Lin H, Chen G, Zhu S, Chen Y, Chen D, Xu W, Yu X, Shi J. The Interaction of CuS and Halothiobacillus HT1 Biofilm in Microscale Using Synchrotron Radiation-Based Techniques. International Journal of Molecular Sciences. 2013; 14(6):11113-11124. https://doi.org/10.3390/ijms140611113
Chicago/Turabian StyleLin, Huirong, Guangcun Chen, Shenhai Zhu, Yingxu Chen, Dongliang Chen, Wei Xu, Xiaohan Yu, and Jiyan Shi. 2013. "The Interaction of CuS and Halothiobacillus HT1 Biofilm in Microscale Using Synchrotron Radiation-Based Techniques" International Journal of Molecular Sciences 14, no. 6: 11113-11124. https://doi.org/10.3390/ijms140611113





