Apoferritin Modified Magnetic Particles as Doxorubicin Carriers for Anticancer Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
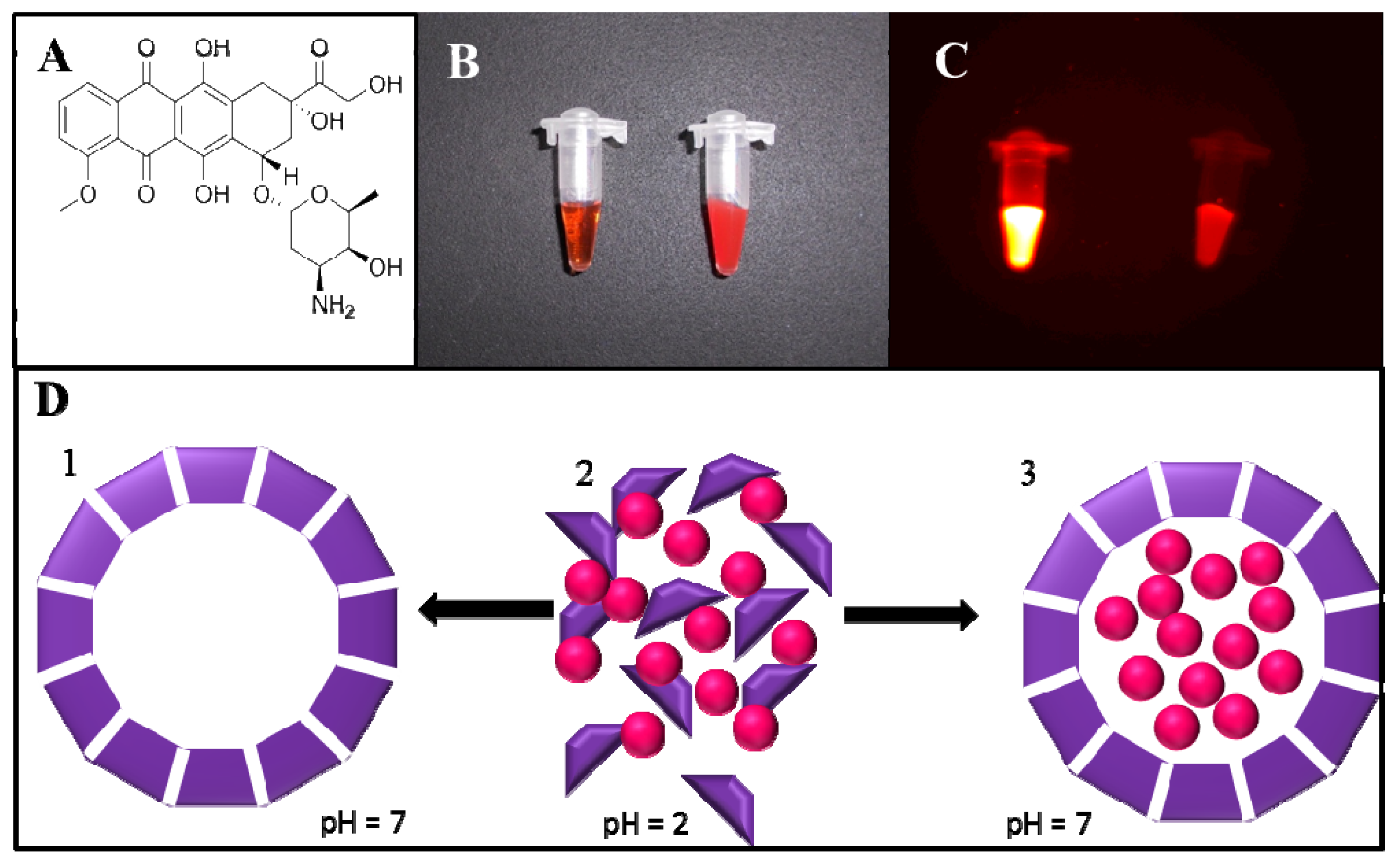
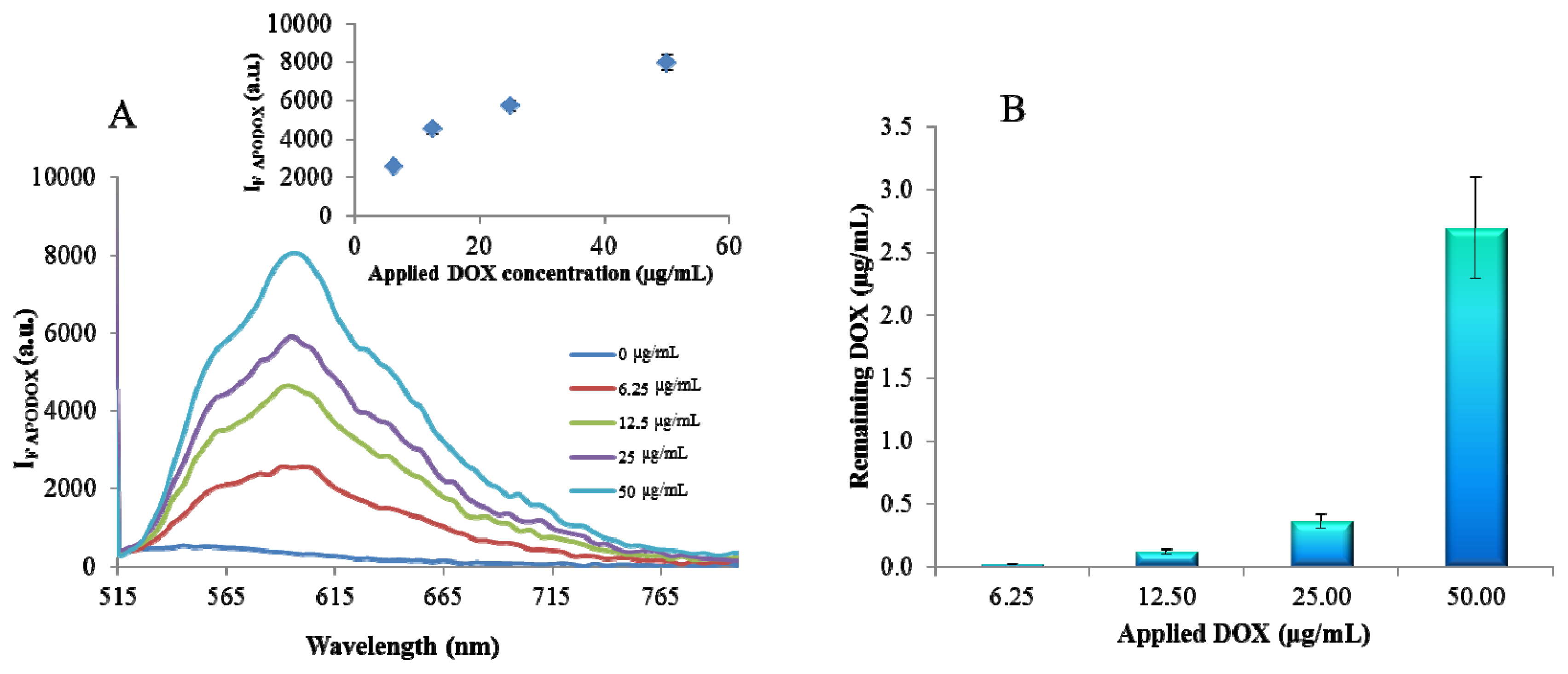
2.1. Fluorimetric Characterization
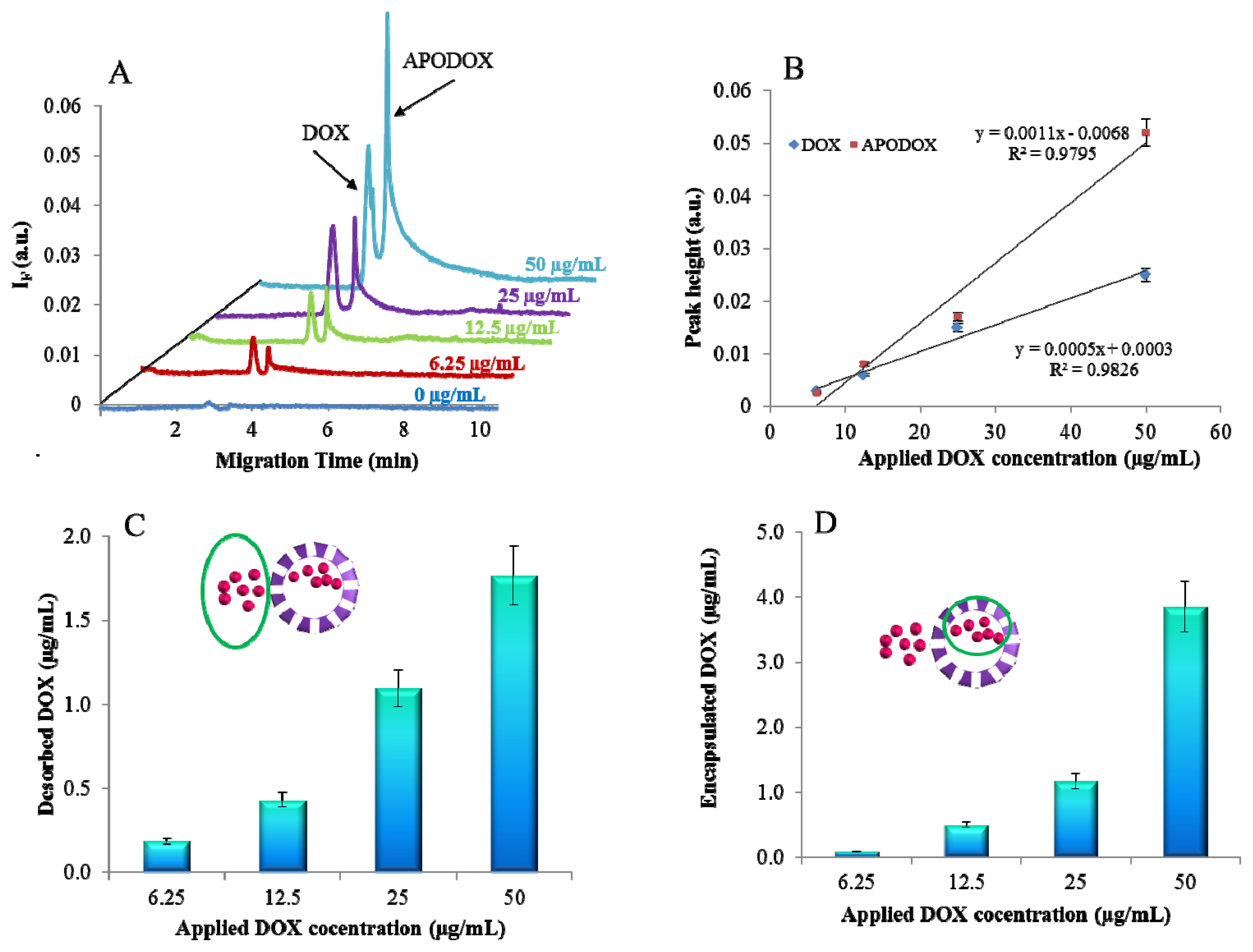
2.2. CE Characterization
2.3. pH Triggered DOX Release
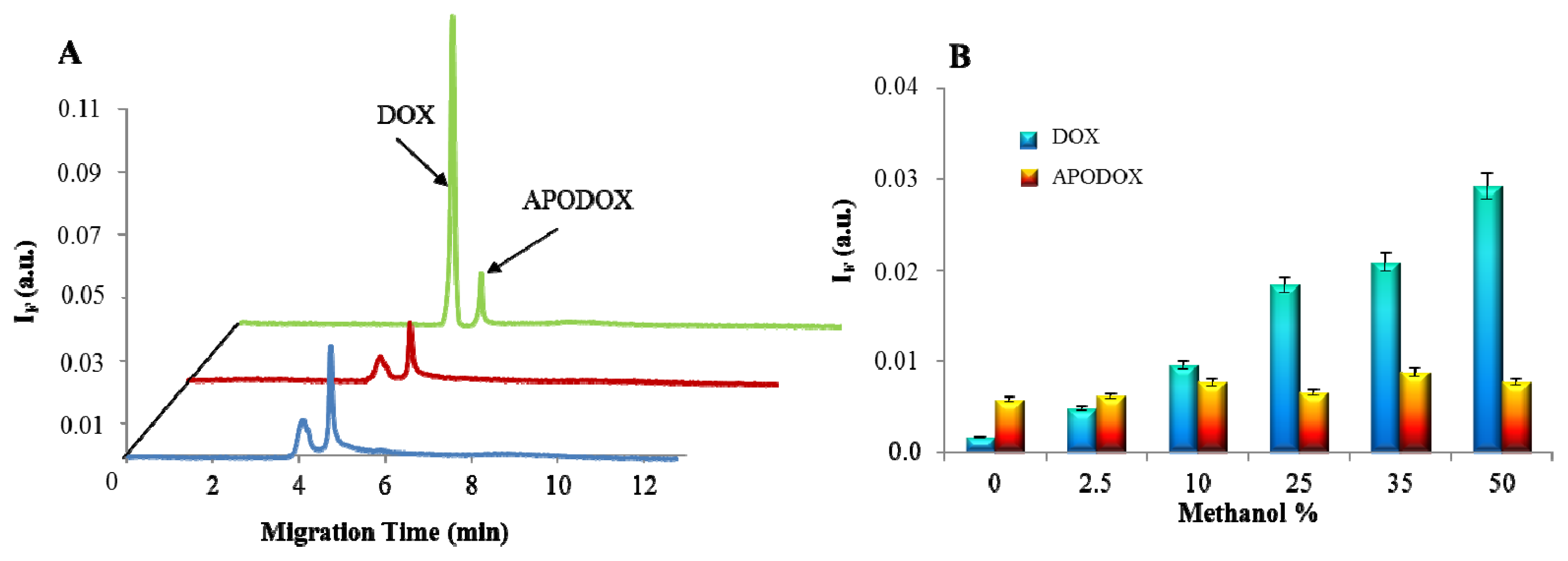
2.4. Influence of MeOH
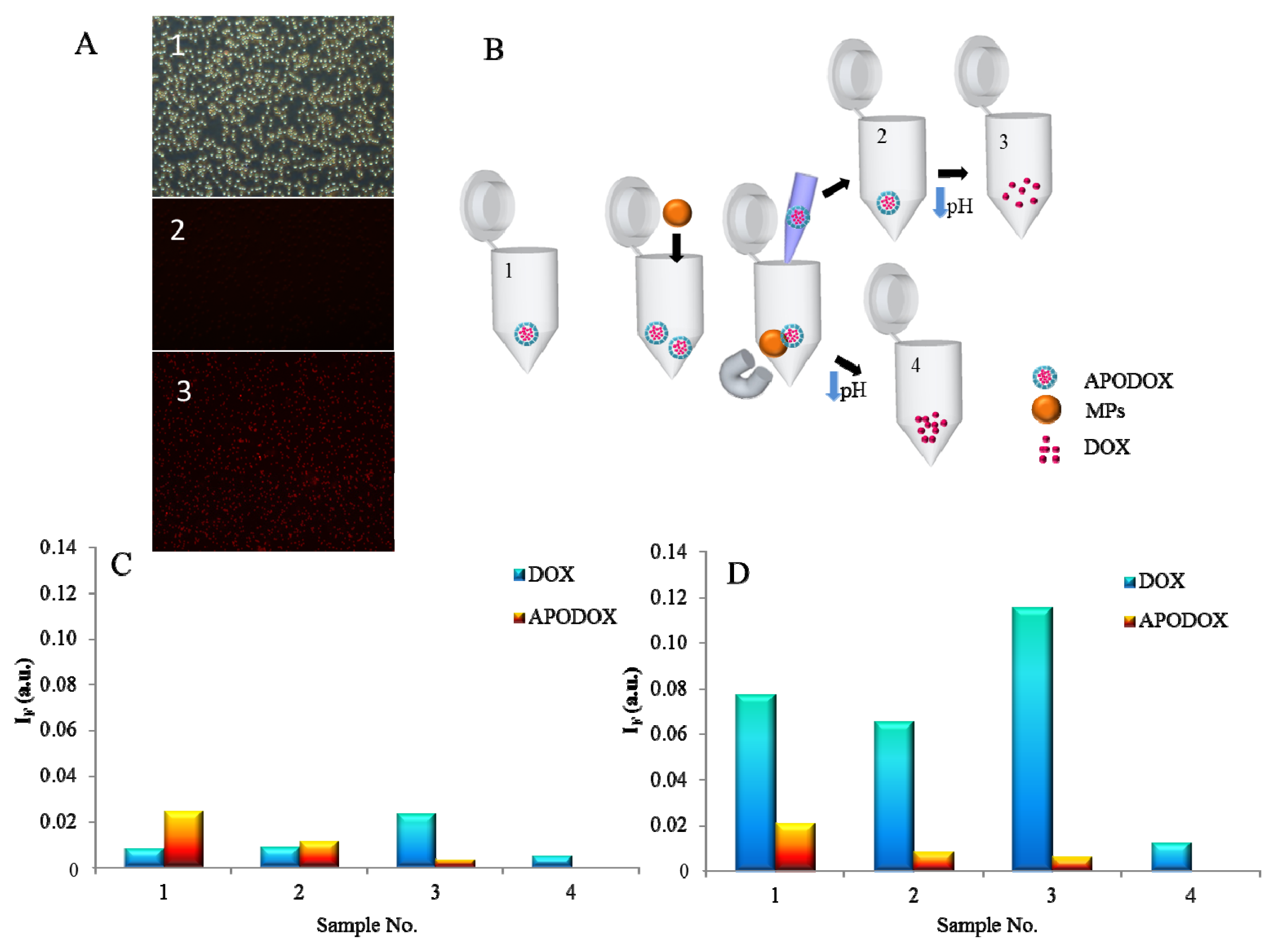
2.5. Magnetic Particle Mediated APODOX Transport
3. Experimental Section
3.1. Apoferritin Encapsulated Doxorubicin (APODOX) Synthesis
3.2. Magnetic Particle Mediated APODOX Transport
3.2.1. Preparation of Biotinylated Apoferritin Filled with Doxorubicin
3.2.2. Modification of Streptavidin-Functionalized Particles with Biotinylated APODOX
3.3. Fluorescent Microscopy
3.4. Fluorimetric Analysis
3.5. Capillary Electrophoresis with Laser-Induced Fluorescence Detection
4. Conclusions
Acknowledgments
Conflict of Interest
References
- El-Okr, M.M.; Salem, M.A.; Salim, M.S.; El-Okr, R.M.; Ashoush, M.; Talaat, H.M. Synthesis of cobalt ferrite nano-particles and their magnetic characterization. J. Magn. Magn. Mater 2011, 323, 920–926. [Google Scholar]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar]
- Nakamura, K.; Ueda, K.; Tomitaka, A.; Yamada, T.; Takemura, Y. Self-heating temperature and AC hysteresis of magnetic iron oxide nanoparticles and their dependence on sary particle size. IEEE Trans. Magn 2013, 49, 240–243. [Google Scholar]
- Nejati, K.; Zabihi, R. Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chem. Cent. J 2012, 6, 1–6. [Google Scholar]
- Thorek, D.L.J.; Tsourkas, A. Size, charge and concentration dependent uptake of iron oxide particles by non-phagocytic cells. Biomaterials 2008, 29, 3583–3590. [Google Scholar]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem 2010, 20, 8760–8767. [Google Scholar]
- Nandori, I.; Racz, J. Magnetic particle hyperthermia: Power losses under circularly polarized field in anisotropic nanoparticles. Phys. Rev. E 2012, 86, 1–8. [Google Scholar]
- Wu, A.G.; Ou, P.; Zeng, L.Y. Biomedical applications of magnetic nanoparticles. Nano 2010, 5, 245–270. [Google Scholar]
- Schlorf, T.; Meincke, M.; Kossel, E.; Gluer, C.C.; Jansen, O.; Mentlein, R. Biological properties of iron oxide nanoparticles for cellular and molecular magnetic resonance imaging. Int. J. Mol. Sci 2011, 12, 12–23. [Google Scholar]
- Nune, S.K.; Gunda, P.; Thallapally, P.K.; Lin, Y.Y.; Forrest, M.L.; Berkland, C.J. Nanoparticles for biomedical imaging. Expert Opin. Drug Deliv 2009, 6, 1175–1194. [Google Scholar]
- Mahmoudi, M.; Simchi, A.; Imani, M. Recent advances in surface engineering of superparamagnetic iron oxide nanoparticles for biomedical applications. J. Iran Chem. Soc 2010, 7, S1–S27. [Google Scholar]
- Peng, X.H.; Qian, X.M.; Mao, H.; Wang, A.Y.; Chen, Z.; Nie, S.M.; Shin, D.M. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int. J. Nanomed 2008, 3, 311–321. [Google Scholar]
- Liu, D.; Zhu, G.L.; Tang, W.Q.; Yang, J.Q.; Guo, H.Y. PCR and magnetic bead-mediated target capture for the isolation of short interspersed nucleotide elements in fishes. Int. J. Mol. Sci 2012, 13, 2048–2062. [Google Scholar]
- Khandare, J.; Minko, T. Polymer-drug conjugates: Progress in polymeric prodrugs. Prog. Polym. Sci 2006, 31, 359–397. [Google Scholar]
- Braconnot, S.; Eissa, M.M.; Elaissari, A. Morphology control of magnetic latex particles prepared from oil in water ferrofluid emulsion. Colloid Polym. Sci 2013, 291, 193–203. [Google Scholar]
- Ding, G.B.; Guo, Y.; Lv, Y.Y.; Liu, X.F.; Xu, L.; Zhang, X.Z. A double-targeted magnetic nanocarrier with potential application in hydrophobic drug delivery. Colloid Surf. B 2012, 91, 68–76. [Google Scholar]
- Eberbeck, D.; Dennis, C.L.; Huls, N.F.; Krycka, K.L.; Gruttner, C.; Westphal, F. Multicore magnetic nanoparticles for magnetic particle imaging. IEEE Trans. Magn 2013, 49, 269–274. [Google Scholar]
- Freund, J.B.; Shapiro, B. Transport of particles by magnetic forces and cellular blood flow in a model microvessel. Phys. Fluids 2012, 24, 1–12. [Google Scholar]
- Mok, H.; Zhang, M.Q. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert Opin. Drug Deliv 2013, 10, 73–87. [Google Scholar]
- Lubbe, A.S.; Bergemann, C.; Riess, H.; Schriever, F.; Reichardt, P.; Possinger, K.; Matthias, M.; Dorken, B.; Herrmann, F.; Gurtler, R.; et al. Clinical experiences with magnetic drag targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res 1996, 56, 4686–4693. [Google Scholar]
- Silva, A.C.; Santos, D.; Ferreira, D.; Lopes, C.M. Lipid-based nanocarriers as an alternative for oral delivery of poorly water-soluble drugs: Peroral and mucosal routes. Curr. Med. Chem 2012, 19, 4495–4510. [Google Scholar]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar]
- Kilic, M.A.; Ozlu, E.; Calis, S. A novel protein-based anticancer drug encapsulating nanosphere: Apoferritin-doxorubicin complex. J. Biomed.Nanotechnol 2012, 8, 508–514. [Google Scholar]
- Banerjee, S.S.; Chen, D.H. A multifunctional magnetic nanocarrier bearing fluorescent dye for targeted drug delivery by enhanced two-photon triggered release. Nanotechnology 2009, 20, 1–10. [Google Scholar]
- Li, L.; ten Hagen, T.L.M.; Schipper, D.; Wijnberg, T.M.; van Rhoon, G.C.; Eggermont, A.M.M.; Lindner, L.H.; Koning, G.A. Triggered content release from optimized stealth thermosensitive liposomes using mild hyperthermia. J. Control. Release 2010, 143, 274–279. [Google Scholar]
- Xu, X.W.; Flores, J.D.; McCormick, C.L. Reversible imine shell cross-linked micelles from aqueous raft-synthesized thermoresponsive triblock copolymers as potential nanocarriers for “pH-Triggered” drug release. Macromolecules 2011, 44, 1327–1334. [Google Scholar]
- Suzumoto, Y.; Okuda, M.; Yamashita, I. Fabrication of zinc oxide semiconductor nanoparticles in the apoferritin cavity. Cryst. Growth Des 2012, 12, 4130–4134. [Google Scholar]
- Wu, F.; Jin, T. Polymer-based sustained-release dosage forms for protein drugs, challenges, and recent advances. AAPS PharmSciTech 2008, 9, 1218–1229. [Google Scholar]
- Li, X.H.; Zhang, Y.H.; Yan, R.H.; Jia, W.X.; Yuan, M.L.; Deng, X.M.; Huang, Z.T. Influence of process parameters on the protein stability encapsulated in poly-dl-lactide-poly(ethylene glycol) microspheres. J. Control Release 2000, 68, 41–52. [Google Scholar]
- Sanchez, A.; Villamayor, B.; Guo, Y.Y.; McIver, J.; Alonso, M.J. Formulation strategies for the stabilization of tetanus toroid in poly(lactide-co-glycolide) microspheres. Int. J. Pharm 1999, 185, 255–266. [Google Scholar]
- Gander, B.; Johansen, P.; NamTran, H.; Merkle, H.P. Thermodynamic approach to protein microencapsulation into poly(d,l-lactide) by spray drying. Int. J. Pharm 1996, 129, 51–61. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Blazkova, I.; Nguyen, H.V.; Dostalova, S.; Kopel, P.; Stanisavljevic, M.; Vaculovicova, M.; Stiborova, M.; Eckschlager, T.; Kizek, R.; Adam, V. Apoferritin Modified Magnetic Particles as Doxorubicin Carriers for Anticancer Drug Delivery. Int. J. Mol. Sci. 2013, 14, 13391-13402. https://doi.org/10.3390/ijms140713391
Blazkova I, Nguyen HV, Dostalova S, Kopel P, Stanisavljevic M, Vaculovicova M, Stiborova M, Eckschlager T, Kizek R, Adam V. Apoferritin Modified Magnetic Particles as Doxorubicin Carriers for Anticancer Drug Delivery. International Journal of Molecular Sciences. 2013; 14(7):13391-13402. https://doi.org/10.3390/ijms140713391
Chicago/Turabian StyleBlazkova, Iva, Hoai Viet Nguyen, Simona Dostalova, Pavel Kopel, Maja Stanisavljevic, Marketa Vaculovicova, Marie Stiborova, Tomas Eckschlager, Rene Kizek, and Vojtech Adam. 2013. "Apoferritin Modified Magnetic Particles as Doxorubicin Carriers for Anticancer Drug Delivery" International Journal of Molecular Sciences 14, no. 7: 13391-13402. https://doi.org/10.3390/ijms140713391




