Sarcosine as a Potential Prostate Cancer Biomarker—A Review
Abstract
:1. Introduction
1.1. History
1.2. Background
2. Methods
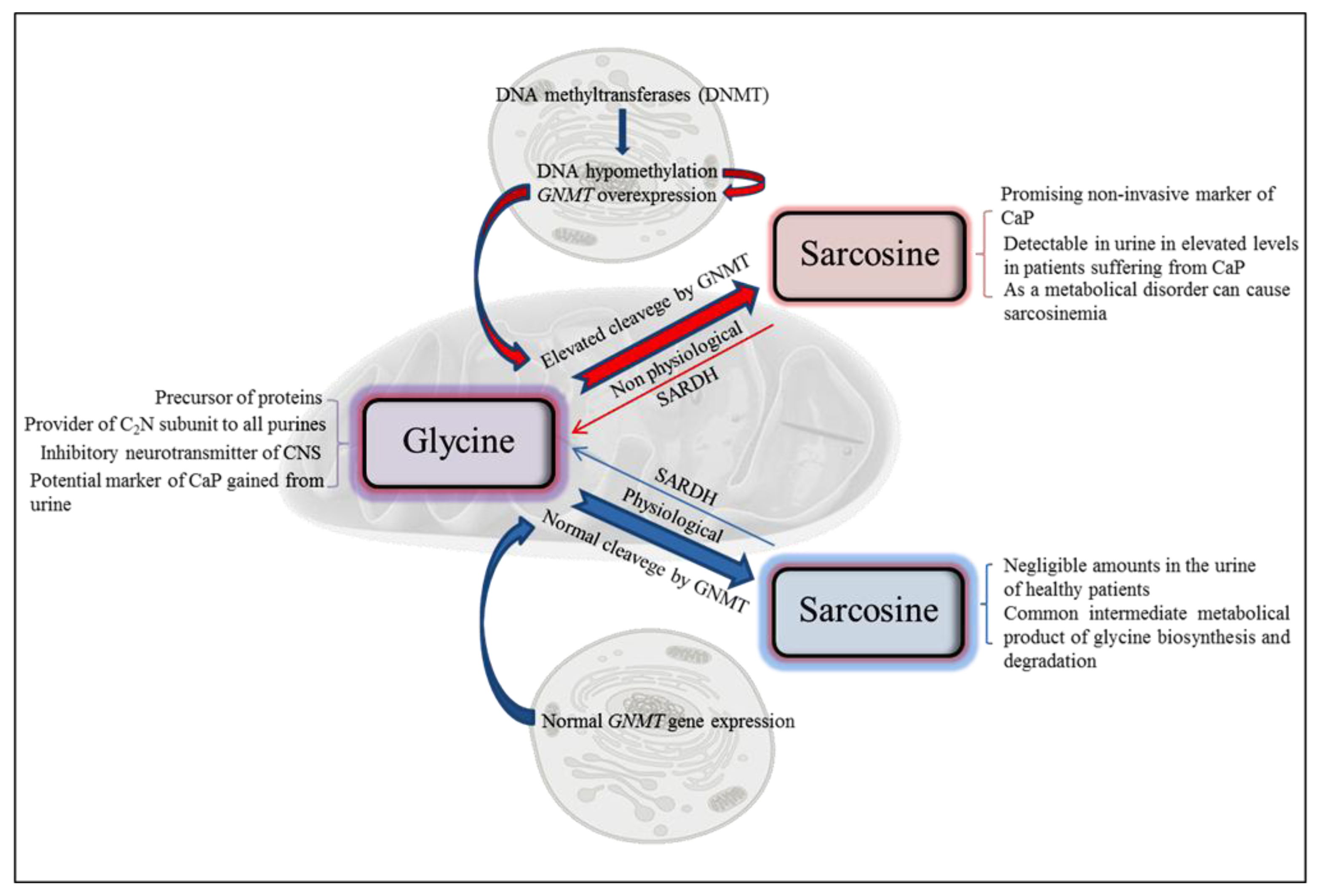
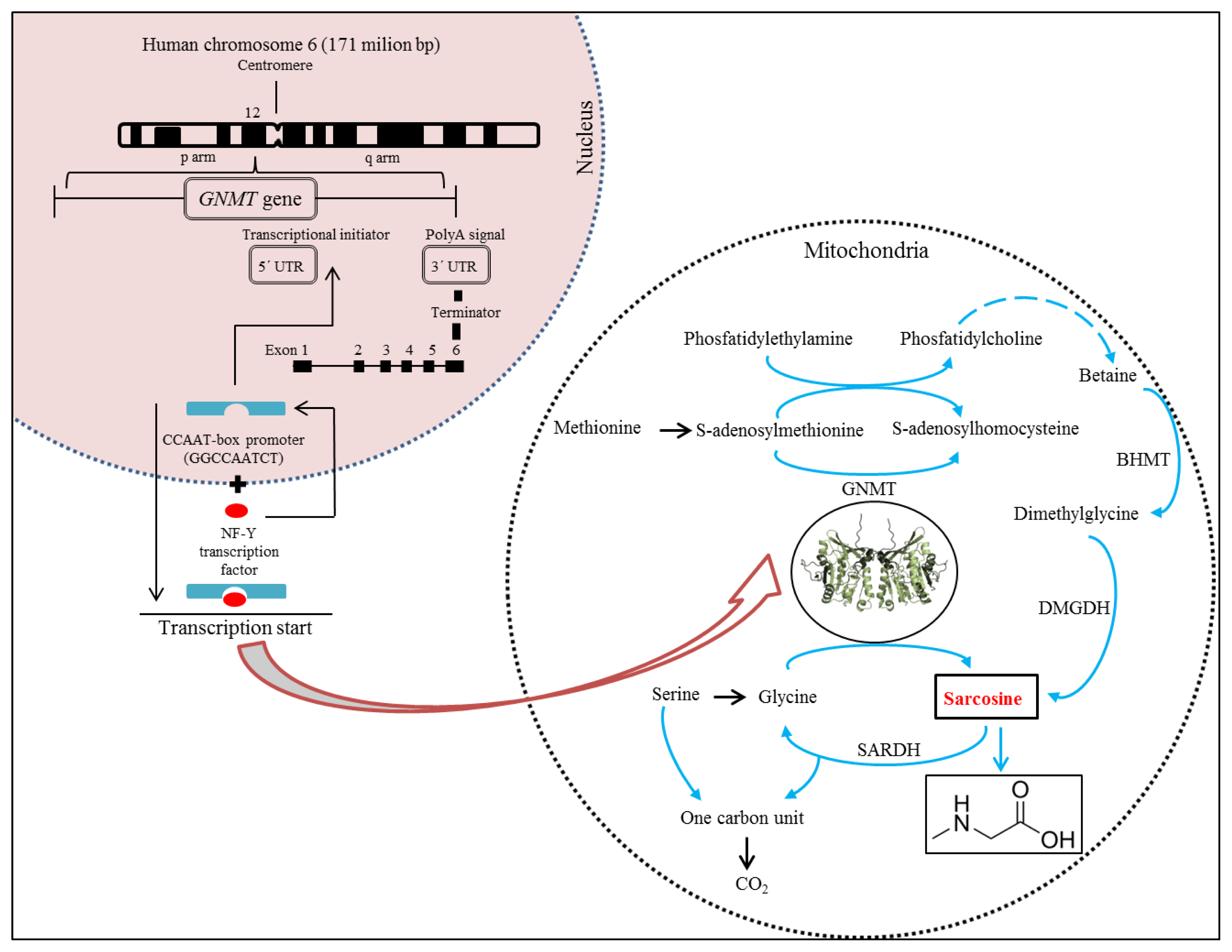
3. Molecular Biology of Sarcosine
4. Metabolism of Sarcosine
5. Metabolomic of Prostate Cancer
6. Analytical Techniques for Detection of Sarcosine
6.1. Microarray-Based Analysis
6.2. Chromatography
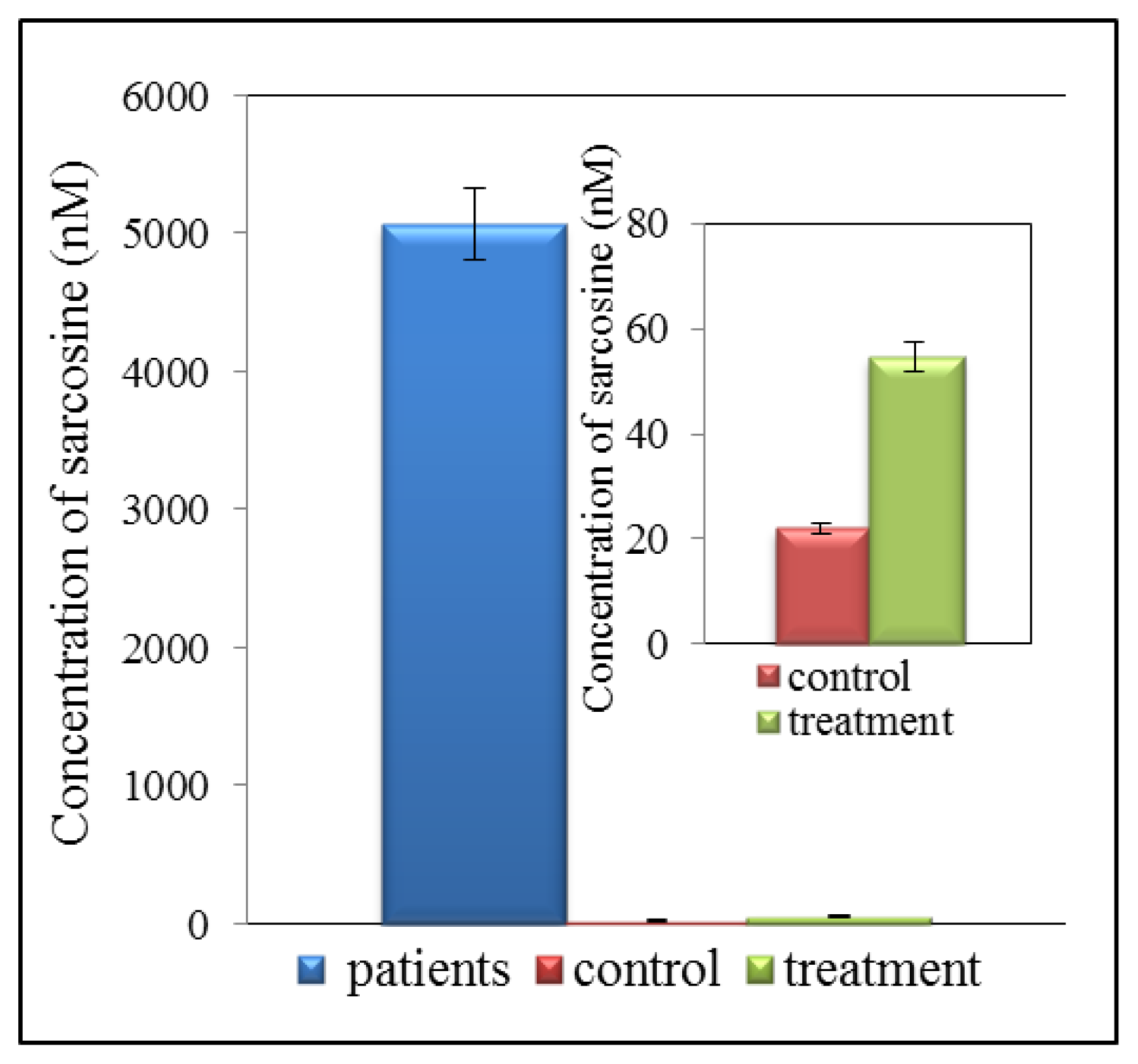
7. Summary of Results Obtained by Analysis of Samples from CaP Patients
8. Conclusions
Acknowledgments
Conflict of Interest
References
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.D.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar]
- Issaq, H.J.; Waybright, T.J.; Veenstra, T.D. Cancer biomarker discovery: Opportunities and pitfalls in analytical methods. Electrophoresis 2011, 32, 967–975. [Google Scholar]
- Cavaliere, B.; Macchione, B.; Monteleone, M.; Naccarato, A.; Sindona, G.; Tagarelli, A. Sarcosine as a marker in prostate cancer progression: A rapid and simple method for its quantification in human urine by solid-phase microextraction-gas chromatography-triple quadrupole mass spectrometry. Anal. Bioanal. Chem 2011, 400, 2903–2912. [Google Scholar]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin 2013, 63, 11–30. [Google Scholar]
- Jamaspishvili, T.; Kral, M.; Khomeriki, I.; Student, V.; Kolar, Z.; Bouchal, J. Urine markers in monitoring for prostate cancer. Prostate Cancer Prostatic Dis 2010, 13, 12–19. [Google Scholar]
- Issaq, H.J.; Veenstra, T.D. Is sarcosine a biomarker for prostate cancer? J. Sep. Sci 2011, 34, 3619–3621. [Google Scholar]
- Jentzmik, F.; Stephan, C.; Lein, M.; Miller, K.; Kamlage, B.; Bethan, B.; Kristiansen, G.; Jung, K. Sarcosine in prostate cancer tissue is not a differential metabolite for prostate cancer aggressiveness and biochemical progression. J. Urol 2011, 185, 706–711. [Google Scholar]
- Palacios, D.A.; Rosser, C.J. Sarcosine, a biomarker for prostate cancer: Ready for prime time? Biomark. Med 2012, 6, 513–514. [Google Scholar]
- Mukherjee, S.; Cruz-Rodriguez, O.; Bolton, E.; Iniguez-Lluhi, J.A. The in vivo role of androgen receptor SUMOylation as revealed by androgen insensitivity syndrome and prostate cancer mutations targeting the proline/glycine residues of synergy control motifs. J. Biol. Chem 2012, 287, 31195–31206. [Google Scholar]
- Hakimi, J.M.; Schoenberg, M.P.; Rondinelli, R.H.; Piantadosi, S.; Barrack, E.R. Androgen receptor variants with short glutamine or glycine repeats may identify unique subpopulations of men with prostate cancer. Clin. Cancer Res 1997, 3, 1599–1608. [Google Scholar]
- Huang, J.; Jiang, L.M.; Ren, P.P.; Zhang, L.M.; Tang, H.R. Comprehensive solid-state NMR analysis reveals the effects of n-methylation on the molecular dynamics of glycine. J. Phys. Chem. B 2012, 116, 136–146. [Google Scholar]
- Patel, S.; Issa, M.M.; El-Galley, R. Evaluation of novel formula of PSA, age, prostate volume, and race in predicting positive prostate biopsy findings. Urology 2013, 81, 602–606. [Google Scholar]
- Morote, J.; Rigau, M.; Garcia, M.; Mir, C.; Ballesteros, C.; Planas, J.; Raventos, C.X.; Placer, J.; de Torres, I.M.; Reventos, J.; et al. Behavior of the PCA3 gene in the urine of men with high grade prostatic intraepithelial neoplasia. World J. Urol 2010, 28, 677–680. [Google Scholar]
- Goode, R.R.; Marshall, S.J.; Duff, M.; Chevli, E.; Chevli, K.K. Use of PCA3 in detecting prostate cancer in initial and repeat prostate biopsy patients. Prostate 2013, 73, 48–53. [Google Scholar]
- Shen, M.; Chen, W.; Yu, K.Y.; Chen, Z.G.; Zhou, W.; Lin, X.M.; Weng, Z.L.; Li, C.D.; Wu, X.L.; Tao, Z.H. The diagnostic value of PCA3 gene-based analysis of urine sediments after digital rectal examination for prostate cancer in a Chinese population. Exp. Mol. Pathol 2011, 90, 97–100. [Google Scholar]
- Wu, N.; Liu, S.Q.; Guo, C.M.; Hou, Z.J.; Sun, M.Z. The role of annexin A3 playing in cancers. Clin. Transl. Oncol 2013, 15, 106–110. [Google Scholar]
- Schostak, M.; Schwall, G.; Poznanovic, S.; Miller, K.; Messinger, D.; Klocker, H.; Stenzl, A.; Feyerabend, S.; Muller, M.; Schrattenholz, A. Annexin A3 quantification from supernatants of urine after dre provides a novel and clinically easy available biomarker for the non-invasive diagnosis of prostate cancer. J. Urol 2007, 177, 470–470. [Google Scholar]
- Schostak, M.; Schwall, G.P.; Poznanovic, S.; Groebe, K.; Muller, M.; Messinger, D.; Miller, K.; Krause, H.; Pelzer, A.; Horninger, W.; et al. Annexin A3 in urine: A highly specific noninvasive marker for prostate cancer early detection. J. Urol 2009, 181, 343–353. [Google Scholar]
- Cao, D.L.; Ye, D.W.; Zhang, H.L.; Zhu, Y.; Wang, Y.X.; Yao, X.D. A multiplex model of combining gene-based, protein-based, and metabolite-based with positive and negative markers in urine for the early diagnosis of prostate cancer. Prostate 2011, 71, 700–710. [Google Scholar]
- Dabir, P.D.; Ottosen, P.; Hoyer, S.; Hamilton-Dutoit, S. Comparative analysis of three- and two-antibody cocktails to AMACR and basal cell markers for the immunohistochemical diagnosis of prostate carcinoma. Diagn. Pathol 2012, 7, 1–6. [Google Scholar]
- Cernei, N.; Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Hrabec, R.; Adam, V.; Kizek, R. Sarcosine in urine of patients with prostate carcinome (in Czech). Praktický Lékař 2012, 92, 444–448. [Google Scholar]
- Fernandez-Serra, A.; Rubio-Briones, J.; Garcia-Casado, Z.; Solsona, E.; Lopez-Guerrero, J.A. Prostate cancer: The revolution of the fusion genes. Actas Urol. Esp 2011, 35, 420–428. [Google Scholar]
- Strittmatter, F.; Stieber, P.; Nagel, D.; Fullhase, C.; Walther, S.; Stief, C.; Waidelich, R. Detection of prostate cancer with complexed PSA and complexed/total PSA ratio: Is there any advantage? Eur. J. Med. Res 2011, 16, 445–450. [Google Scholar]
- Filella, X.; Gimenez, N. Evaluation of [−2]proPSA and Prostate Health Index (phi) for the detection of prostate cancer: A systematic review and meta-analysis. Clin. Chem. Lab. Med 2013, 51, 729–739. [Google Scholar]
- Tosoian, J.J.; Loeb, S.; Feng, Z.Y.; Isharwal, S.; Landis, P.; Elliot, D.J.; Veltri, R.; Epstein, J.I.; Partin, A.W.; Carter, H.B.; et al. Association of [−2]proPSA with biopsy reclassification during active surveillance for prostate cancer. J. Urol 2012, 188, 1131–1136. [Google Scholar]
- Vesprini, D.; Liu, S.; Nam, R. Predicting high risk disease using serum and DNA biomarkers. Curr. Opin. Urol 2013, 23, 252–260. [Google Scholar]
- Pin, E.; Fredolini, C.; Petricoin, E.F. The role of proteomics in prostate cancer research: Biomarker discovery and validation. Clin. Biochem 2013, 46, 524–538. [Google Scholar]
- Ryan, D.; Robards, K.; Prenzler, P.D.; Kendall, M. Recent and potential developments in the analysis of urine: A review. Anal. Chim. Acta 2011, 684, 17–29. [Google Scholar]
- Wang, Y.C.; Chen, Y.M.; Lin, Y.J.; Liu, S.P.; Chiang, E.P.I. GNMT expression increases hepatic folate contents and folate-dependent methionine synthase-mediated homocysteine remethylation. Mol. Med 2011, 17, 486–494. [Google Scholar]
- Yen, C.H.; Lin, Y.T.; Chen, H.L.; Chen, S.Y.; Chen, Y.M.A. The multi-functional roles of GNMT in toxicology and cancer. Toxicol. Appl. Pharmacol 2013, 266, 67–75. [Google Scholar]
- Soriano, A.; Castillo, R.; Christov, C.; Andres, J.; Moliner, V.; Tunon, I. Catalysis in glycine N-methyltransferase: Testing the electrostatic stabilization and compression hypothesis. Biochemistry 2006, 45, 14917–14925. [Google Scholar]
- Velichkova, P.; Himo, F. Methyl transfer in glycine N-methyltransferase. A theoretical study. J. Phys. Chem. B 2005, 109, 8216–8219. [Google Scholar]
- Song, Y.H.; Shiota, M.; Kuroiwa, K.; Naito, S.; Oda, Y. The important role of glycine N-methyltransferase in the carcinogenesis and progression of prostate cancer. Mod. Pathol 2011, 24, 1272–1280. [Google Scholar]
- Ianni, M.; Porcellini, E.; Carbone, I.; Potenzoni, M.; Pieri, A.M.; Pastizzaro, C.D.; Benecchi, L.; Licastro, F. Genetic factors regulating inflammation and DNA methylation associated with prostate cancer. Prostate Cancer Prostatic Dis 2013, 16, 56–60. [Google Scholar]
- Huang, Y.C.; Lee, C.M.; Chen, M.; Chung, M.Y.; Chang, Y.H.; Huang, W.J.S.; Ho, D.M.T.; Pan, C.C.; Wu, T.T.; Yang, S.; et al. Haplotypes, loss of heterozygosity, and expression levels of glycine N-methyltransferase in prostate cancer. Clin. Cancer Res 2007, 13, 1412–1420. [Google Scholar]
- Lee, C.M.; Shih, Y.P.; Wu, C.H.; Chen, Y.M.A. Characterization of the 5′ regulatory region of the human Glycine N-methyltransferase gene. Gene 2009, 443, 151–157. [Google Scholar]
- Mantovani, R. The molecular biology of the CCAAT-binding factor NF-Y. Gene 1999, 239, 15–27. [Google Scholar]
- Nardini, M.; Gnesutta, N.; Donati, G.; Gatta, R.; Forni, C.; Fossati, A.; Vonrhein, C.; Moras, D.; Romier, C.; Bolognesi, M.; et al. Sequence-specific transcription factor NF-Y displays histone-like DNA binding and H2B-like ubiquitination. Cell 2013, 152, 132–143. [Google Scholar]
- Petroni, K.; Kumimoto, R.W.; Gnesutta, N.; Calvenzani, V.; Fornari, M.; Tonelli, C.; Holt, B.F.; Mantovani, R. The promiscuous life of plant nuclear factor Y transcription factors. Plant Cell 2012, 24, 4777–4792. [Google Scholar]
- Dolfini, D.; Minuzzo, M.; Pavesi, G.; Mantovani, R. The short isoform of NF-YA belongs to the embryonic stem cell transcription factor circuitry. Stem Cells 2012, 30, 2450–2459. [Google Scholar]
- Calvenzani, V.; Testoni, B.; Gusmaroli, G.; Lorenzo, M.; Gnesutta, N.; Petroni, K.; Mantovani, R.; Tonelli, C. Interactions and CCAAT-binding of arabidopsis thaliana NF-Y subunits. PLoS One 2012, 7, 1–11. [Google Scholar]
- Luka, Z.; Cerone, R.; Phillips, J.A.; Mudd, S.H.; Wagner, C. Mutations in human glycine N-methyltransferase give insights into its role in methionine metabolism. Hum. Genet 2002, 110, 68–74. [Google Scholar]
- Liao, Y.J.; Liu, S.P.; Lee, C.M.; Yen, C.H.; Chuang, P.C.; Chen, C.Y.; Tsai, T.F.; Huang, S.F.; Lee, Y.H.W.; Chen, Y.M.A. Characterization of a glycine N-methyltransferase gene knockout mouse model for hepatocellular carcinoma: Implications of the gender disparity in liver cancer susceptibility. Int. J. Cancer 2009, 124, 816–826. [Google Scholar]
- Stabler, S.; Koyama, T.; Zhao, Z.G.; Martinez-Ferrer, M.; Allen, R.H.; Luka, Z.; Loukachevitch, L.V.; Clark, P.E.; Wagner, C.; Bhowmick, N.A. Serum methionine metabolites are risk factors for metastatic prostate cancer progression. PLoS One 2011, 6, e22486. [Google Scholar]
- Dahl, M.; Bouchelouche, P.; Kramer-Marek, G.; Capala, J.; Nordling, J.; Bouchelouche, K. Sarcosine induces increase in HER2/neu expression in androgen-dependent prostate cancer cells. Mol. Biol. Rep 2011, 38, 4237–4243. [Google Scholar]
- Metallo, C.M. Expanding the reach of cancer metabolomics. Cancer Prev. Res 2012, 5, 1337–1340. [Google Scholar]
- Burton, C.; Gamagedara, S.; Ma, Y.F. A novel enzymatic technique for determination of sarcosine in urine samples. Anal. Methods 2012, 4, 141–146. [Google Scholar]
- Montrose, D.C.; Zhou, X.K.; Kopelovich, L.; Yantiss, R.K.; Karoly, E.D.; Subbaramaiah, K.; Dannenberg, A.J. Metabolic profiling, a noninvasive approach for the detection of experimental colorectal neoplasia. Cancer Prev. Res 2012, 5, 1358–1367. [Google Scholar]
- Luka, Z.; Pakhomova, S.; Loukachevitch, L.V.; Newcomer, M.E.; Wagner, C. Differences in folate-protein interactions result in differing inhibition of native rat liver and recombinant glycine N-methyltransferase by 5-methyltetrahydrofolate. Bba Proteins Proteomics 2012, 1824, 286–291. [Google Scholar]
- Mitchell, A.D.; Benevenga, N.J. Importance of sarcosine formation in methionine methyl carbon oxidation in rat. J. Nutr 1976, 106, 1702–1713. [Google Scholar]
- Bar-joseph, I.; Pras, E.; Reznik-Wolf, H.; Marek-Yagel, D.; Abu-Horvitz, A.; Dushnitzky, M.; Goldstein, N.; Rienstein, S.; Dekel, M.; Pode-Shakked, B.; et al. Mutations in the sarcosine dehydrogenase gene in patients with sarcosinemia. Hum. Genet 2012, 131, 1805–1810. [Google Scholar]
- Bergeron, F.; Otto, A.; Blache, P.; Day, R.; Denoroy, L.; Brandsch, R.; Bataille, D. Molecular cloning and tissue distribution of rat sarcosine dehydrogenase. Eur. J. Biochem 1998, 257, 556–561. [Google Scholar]
- Trock, B.J. Application of metabolomics to prostate cancer. Urol. Oncol. Semin. Orig. Investig 2011, 29, 572–581. [Google Scholar]
- Spratlin, J.L.; Serkova, N.J.; Eckhardt, S.G. Clinical applications of metabolomics in oncology: A review. Clin. Cancer Res 2009, 15, 431–440. [Google Scholar]
- Kami, K.; Fujimori, T.; Sato, H.; Sato, M.; Yamamoto, H.; Ohashi, Y.; Sugiyama, N.; Ishihama, Y.; Onozuka, H.; Ochiai, A.; et al. Metabolomic profiling of lung and prostate tumor tissues by capillary electrophoresis time-of-flight mass spectrometry. Metabolomics 2013, 9, 444–453. [Google Scholar]
- Cheng, L.L.; Wu, C.; Smith, M.R.; Gonzalez, R.G. Non-destructive quantitation of spermine in human prostate tissue samples using HRMAS 1H-NMR spectroscopy at 9.4 T. FEBS Lett 2001, 494, 112–116. [Google Scholar]
- Swanson, M.G.; Zektzer, A.S.; Tabatabai, Z.L.; Simko, J.; Jarso, S.; Keshari, K.R.; Schmitt, L.; Carroll, P.R.; Shinohara, K.; Vigneron, D.B.; et al. Quantitative analysis of prostate metabolites using 1H HR-MAS spectroscopy. Magn. Reson. Med 2006, 55, 1257–1264. [Google Scholar]
- DeFeo, E.M.; Wu, C.L.; McDougal, W.S.; Cheng, L.L. A decade in prostate cancer: From NMR to metabolomics. Nat. Rev. Urol 2011, 8, 301–311. [Google Scholar]
- Lucarelli, G.; Fanelli, M.; Larocca, A.M.V.; Germinario, C.A.; Rutigliano, M.; Vavallo, A.; Selvaggi, F.P.; Bettocchi, C.; Battaglia, M.; Ditonno, P. Serum sarcosine increases the accuracy of prostate cancer detection in patients with total serum PSA less than 4.0 ng/mL. Prostate 2012, 72, 1611–1621. [Google Scholar]
- Shaughnessy, J.D.; Barlogie, B. Interpreting the molecular biology and clinical behavior of multiple myeloma in the context of global gene expression profiling. Immunol. Rev 2003, 194, 140–163. [Google Scholar]
- Bohm, L.; Serafin, A.M.; Fernandez, P.; van der Watt, G.; Bouic, P.J.D.; Harvey, J. Plasma sarcosine does not distinguish early and advanced stages of prostate cancer. SAMJ S. Afr. Med. J 2012, 102, 677–679. [Google Scholar]
- Chen, J.; Zhou, L.N.; Zhang, X.Y.; Lu, X.; Cao, R.; Xu, C.J.; Xu, G.W. Urinary hydrophilic and hydrophobic metabolic profiling based on liquid chromatography-mass spectrometry methods: Differential metabolite discovery specific to ovarian cancer. Electrophoresis 2012, 33, 3361–3369. [Google Scholar]
- Schuller, D.J.; Reisch, C.R.; Moran, M.A.; Whitman, W.B.; Lanzilotta, W.N. Structures of dimethylsulfoniopropionate-dependent demethylase from the marine organism Pelagabacter ubique. Protein Sci 2012, 21, 289–298. [Google Scholar]
- Cernei, N.; Masarik, M.; Gumulec, J.; Zitka, O.; Babula, P.; Kizek, R. Determination of sarcosine as possible tumour marker of prostate tumours. J. Biochem. Techol 2010, 2, S7–S8. [Google Scholar]
- Jiang, Y.Q.; Cheng, X.L.; Wang, C.A.; Ma, Y.F. Quantitative determination of sarcosine and related compounds in urinary samples by liquid chromatography with tandem mass spectrometry. Anal. Chem 2010, 82, 9022–9027. [Google Scholar]
- Cernei, N.; Zitka, O.; Ryvolova, M.; Adam, V.; Masarik, M.; Hubalek, J.; Kizek, R. Spectrometric and electrochemical analysis of sarcosine as a potential prostate carcinoma marker. Int. J. Electrochem. Sci 2012, 7, 4286–4301. [Google Scholar]
- LeBoucher, J.; Charret, C.; CoudrayLucas, C.; Giboudeau, J.; Cynober, L. Amino acid determination in biological fluids by automated ion-exchange chromatography: Performance of Hitachi L-8500A. Clin. Chem 1997, 43, 1421–1428. [Google Scholar]
- Khan, A.P.; Rajendiran, T.M.; Ateeq, B.; Asangani, I.A.; Athanikar, A.R.; Yocum, A.K.; Mehra, R.; Siddiqui, J.; Palapattu, G.; Wei, J.T.; et al. The role of sarcosine metabolism in prostate cancer progression. Neoplasia 2013, 15, 491–501. [Google Scholar]
- Jentzmik, F.; Stephan, C.; Miller, K.; Schrader, M.; Erbersdobler, A.; Kristiansen, G.; Lein, M.; Jung, K. Sarcosine in urine after digital rectal examination fails as a marker in prostate cancer detection and identification of aggressive tumours. Eur. Urol. 2010, 58, 12–18. [Google Scholar]
- Struys, E.A.; Heijboer, A.C.; van Moorselaar, J.; Jakobs, C.; Blankenstein, M.A. Serum sarcosine is not a marker for prostate cancer. Ann. Clin. Biochem 2010, 47, 282–282. [Google Scholar]
- Wu, H.; Liu, T.T.; Ma, C.G.; Xue, R.Y.; Deng, C.H.; Zeng, H.Z.; Shen, X.Z. GC/MS-based metabolomic approach to validate the role of urinary sarcosine and target biomarkers for human prostate cancer by microwave-assisted derivatization. Anal. Bioanal. Chem 2011, 401, 635–646. [Google Scholar]
- Bianchi, F.; Dugheri, S.; Musci, M.; Bonacchi, A.; Salvadori, E.; Arcangeli, G.; Cupelli, V.; Lanciotti, M.; Masieri, L.; Serni, S.; et al. Fully automated solid-phase microextraction-fast gas chromatography-mass spectrometry method using a new ionic liquid column for high-throughput analysis of sarcosine and N-ethylglycine in human urine and urinary sediments. Anal. Chim. Acta 2011, 707, 197–203. [Google Scholar]
- Cao, D.L.; Ye, D.W.; Zhu, Y.; Zhang, H.L.; Wang, Y.X.; Yao, X.D. Efforts to resolve the contradictions in early diagnosis of prostate cancer: A comparison of different algorithms of sarcosine in urine. Prostate Cancer Prostatic Dis 2011, 14, 166–172. [Google Scholar]

| Method | Matrix | Sample pre-treatment | Ref. |
|---|---|---|---|
| Microarray-based analysis | DNA | Medium | [5] |
| GC/MS | Urine, tissue, serum | High | [61] |
| LC/MS | Urine, tissue, serum | High | [65] |
| LC/ED | Urine, tissue, serum | High | [21] |
| IEC | Urine, tissue, serum | High | [21] |
| Number of patients | Diagnosis | Sarcosine | Ref. |
|---|---|---|---|
| 10 | Non-specific prostate cancer | + | [47] |
| 29 | Low differentiated acinar prostate adenocarcinoma | + | [21] |
| 14 | Metastatic prostate cancer | + | [1] |
| 71 | Medium differentiated prostate acinar adenocarcinoma | + | [21] |
| 106 | Non-specific prostate cancer | − | [69] |
| 15 | Non-specific prostate cancer | + | [71] |
| 10 | Acinar adenocarcinoma of prostate | + | [21] |
| 33 | Metastatic prostate cancer | + | [72] |
| 86 | Non-specific prostate cancer | + | [19] |
| 13 | Medium differentiated prostate acinar adenocarcinoma | + | [73] |
| 3 | Metastatic prostate cancer | + | [65] |
| 18 | Metastatic prostate cancer | + | [70] |
| 290 | Metastatic prostate cancer | + | [59] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cernei, N.; Heger, Z.; Gumulec, J.; Zitka, O.; Masarik, M.; Babula, P.; Eckschlager, T.; Stiborova, M.; Kizek, R.; Adam, V. Sarcosine as a Potential Prostate Cancer Biomarker—A Review. Int. J. Mol. Sci. 2013, 14, 13893-13908. https://doi.org/10.3390/ijms140713893
Cernei N, Heger Z, Gumulec J, Zitka O, Masarik M, Babula P, Eckschlager T, Stiborova M, Kizek R, Adam V. Sarcosine as a Potential Prostate Cancer Biomarker—A Review. International Journal of Molecular Sciences. 2013; 14(7):13893-13908. https://doi.org/10.3390/ijms140713893
Chicago/Turabian StyleCernei, Natalia, Zbynek Heger, Jaromir Gumulec, Ondrej Zitka, Michal Masarik, Petr Babula, Tomas Eckschlager, Marie Stiborova, Rene Kizek, and Vojtech Adam. 2013. "Sarcosine as a Potential Prostate Cancer Biomarker—A Review" International Journal of Molecular Sciences 14, no. 7: 13893-13908. https://doi.org/10.3390/ijms140713893







