Secondary Plant Products Causing Photosensitization in Grazing Herbivores: Their Structure, Activity and Regulation
Abstract
:1. Introduction: Bioactive Secondary Products Produced by Plants—Their Role in Plant Defense
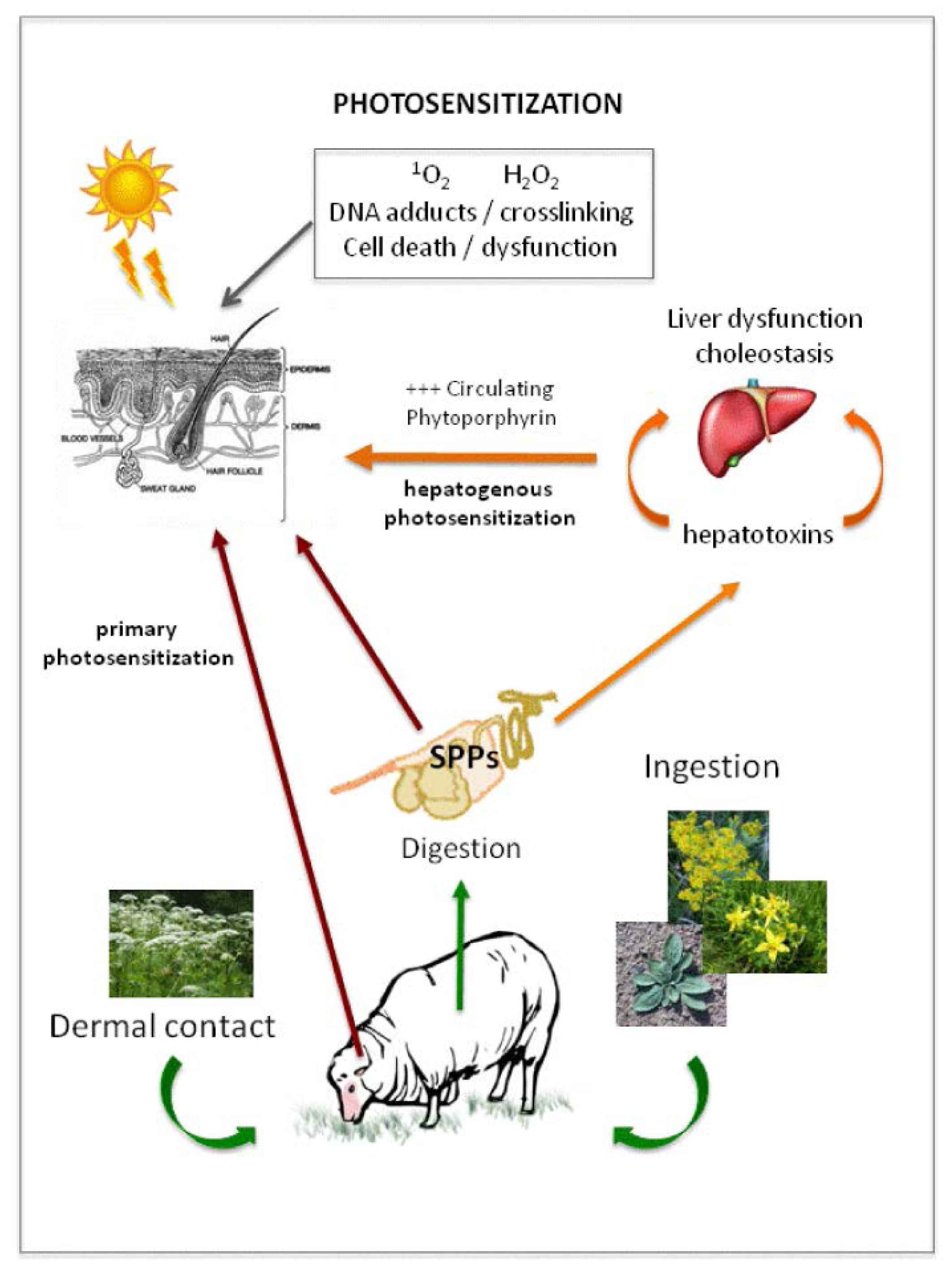
2. Photosensitizing Plant Compounds Affect Grazing Herbivores
3. The Role of Light Reactive Molecules in the Photosensitization Process
3.1. Production of Photosensitizers in Higher Plants
3.2. Regulation and Expression of Photosensitizers in Higher Plants
4. Direct and Indirect Effects of Plant-Derived Light-Reactive Molecules in Animal Systems
4.1. Photosensitization in Animals
4.2. Delivery of Light Reactive Compounds to Animal Systems Resulting in Photosensitization
4.3. Factors Influencing Sensitivity of Animals to Light Reactive Compounds Causing Photosensitization
5. Case Studies Documenting Photosensitization in Grazing Herbivores Due to Exposure to Light Reactive Plant-Produced Compounds
5.1. Primary Contact Photosensitization and Giant Hogweed (Heracleum mantegazzianum) Sommier and Levier
Primary Ingestion Photosensitization and St. John’s Wort (Hypericum perforatum L.)
5.2. Secondary Photosensitization: Hepatogenic Dysfunction in Grazing Herbivores
5.2.1. The Role of the Chlorophyll Metabolite Phytoporphyrin in Hepatogenic Photosensitazion
5.2.2. Lantana spp. and the Triterpenes
5.2.3. The Steroidal Saponins: Switchgrass (Panicum spp.) (Poaceae)
5.2.4. Photosensitization and Pyrrolizidine Alkaloids: Paterson’s Curse (Echium plantagineum L.) and Senecio spp
5.2.4.1. Echium spp
5.2.4.2. Senecio spp
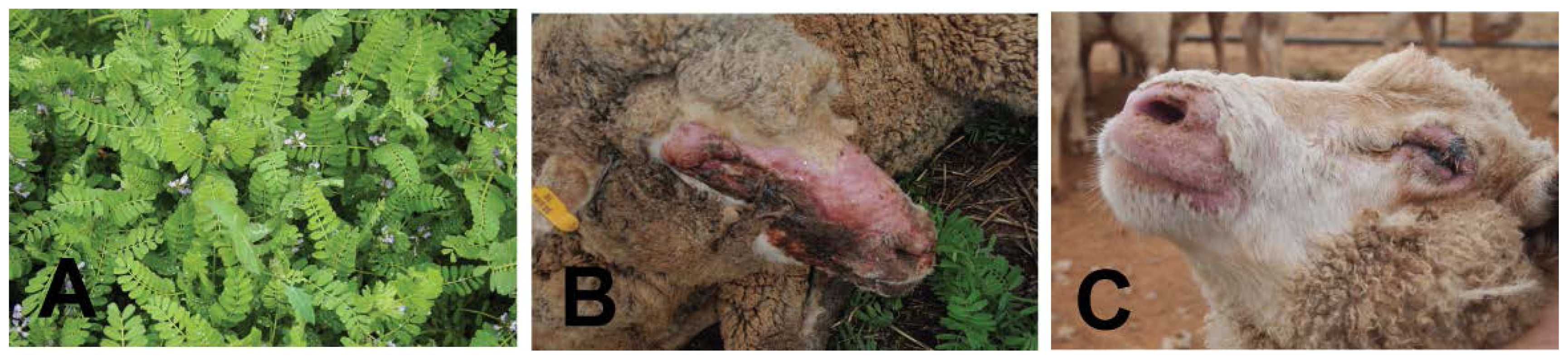
5.3. Biserrula (Biserrula pelecinus)—A Novel Photosensitizing Plant
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Croteau, R.; Kutchman, T.M.; Lewis, N.G. Natural products (secondary metabolites). In Biochemistry and Molecular Biology of Plants; American Society of Plant Biologists: Rockville, MD, USA, 2000. [Google Scholar]
- Field, B.; Jordan, F.; Osbourn, A. First encounters—Deployment of defence-related natural products by plants. N. Phytol 2006, 172, 193–207. [Google Scholar]
- Weston, P.A.; Weston, L.A.; Hildebrand, P.A. Metabolic profiling in Echium plantagineum: Presence of bioactive pyrrolizidine alkaloids and naphthoquinones from accenssions across Australia. Phytochem. Rev 2013, 12, 831–837. [Google Scholar]
- Wink, M. Functions and biotechnology of plant secondary metabolites. Ann. Plant Rev 1999, 39, 1–16. [Google Scholar]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol 2013, 39, 283–297. [Google Scholar]
- Ferenc, P.; Solar, P.; Kleban, J.; Mikes, J.; Fedorocko, P. Down-regulation of Bcl-2 and Akt induced by combination of photoactivated hypericin and genistein in human breast cancer cells. J. Photochem. Photobiol 2010, 98, 25–34. [Google Scholar]
- Pickett, J.A. New synthesis: Chemical ecology and sustainable food production. J. Chem. Ecol 2012, 38, 1071–1071. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Potential of Allelopathy and Allelochemicals for Weed Management; Food Products Press: Binghamton, NY, USA, 2006. [Google Scholar]
- Romeo, J.T. Special Issue: Allelochemical Interactions in Agro- and natural ecosystems preface. J. Chem. Ecol 2013, 39, 141. [Google Scholar]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci 2003, 22, 367–389. [Google Scholar]
- Berenbaum, M.; Feeny, P. Toxicity of angular furanocoumarins to swallowtail butterflies—Escalation in a co-evolutionary arms-race. Science 1981, 212, 927–929. [Google Scholar]
- Pfister, J.A. Behavioural Strategies for Coping with Poisonous Plants; University of Idaho: Moscow, ID, USA, 1999. [Google Scholar]
- Provenza, F.D.; Pfister, J.A.; Cheney, C.D. Mechanisms of learning in diet selection with reference to phytotoxicosis in herbivores. J. Range Manag 1992, 45, 36–45. [Google Scholar]
- Launchbaugh, K.L. Biochemical Aspects of Grazing Behavior; CAB International: Wallingford, Oxfordshire, UK, 1996; pp. 159–184. [Google Scholar]
- Gupta, R.C. Veterinary Toxicology—Basic and Clinical Principles, 2nd ed; Elseiver: London, UK, 2012. [Google Scholar]
- Rowe, L.D. Photosensitization problems in livestock. Vet. Clin. N. Am. Food Anim. Pract 1989, 5, 301–323. [Google Scholar]
- McKenzie, R. Australia’s Poisonous Plants, Fungi and Cyanobacteria; CSIRO Publishing: Collingwood, Australia, 2012. [Google Scholar]
- Spikes, J.D. Photosensitization, 2nd ed; Plenum Press: New York, NY, USA, 1989. [Google Scholar]
- Knight, A.P.; Walter, R.G. A Guide to Plant Poisoning of Animals in North America; Teton New Media: Jackson, WY, USA, 2001. [Google Scholar]
- Smith, E.; Kiss, F.; Porter, R.M.; Anstey, A.V. A review of UVA-mediated photosensitivity disorders. Photochem. Photobiol. Sci 2012, 11, 199–206. [Google Scholar]
- Yamori, W.; Sakata, N.; Suzuki, Y.; Shikanai, T.; Makino, A. Cyclic electron flow around photosystem I via chloroplast NAD(P)H dehydrogenase (NDH) complex performs a significant physiological role during photosynthesis and plant growth at low temperature in rice. Plant J 2011, 68, 966–976. [Google Scholar]
- Yamori, W. Improving photosynthesis to increase food and fuel production by biotechnological strategies in crops. J. Plant Biochem. Physiol 2013, 1, 113. [Google Scholar]
- Wallaart, T.E.; Pras, N.; Quax, W.J. Isolation and identification of dihydroartemisinic acid hydroperoxide from Artemisia annua: A novel biosynthetic precursor of artemisinin. J. Nat. Prod 1999, 62, 1160–1162. [Google Scholar]
- Oleinick, N.L. Basic photosensitization. (accessed on 15 December 2013).
- Gohare, A.; Greer, S. Type I and Type II Photosensitized Oxidation in Chemistry and Biology. Photochem. Photobiol 2012. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Cuine, S.; Triantaphylides, C.; Ravanat, J.L.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol 2012, 158, 1267–1278. [Google Scholar]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylides, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar]
- Alscher, R.G.; Donahue, J.L.; Cramer, C.L. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plantarum 1997, 100, 224–233. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol 2004, 55, 373–399. [Google Scholar]
- Wallaart, T.E.; Pras, N.; Quax, W.J. Seasonal variations of artemisinin and its biosynthetic precursors in tetraploid Artemisia annua plants compared with the diploid wild-type. Planta Med 1999, 65, 723–728. [Google Scholar]
- Theodossiou, T.A.; Hothersall, J.S.; De Witte, P.A.; Pantos, A.; Agostinis, P. The multifaceted photocytotoxic profile of hypericin. Mol. Pharm 2009, 6, 1775–1789. [Google Scholar]
- Brechner, M.L.; Albright, L.D.; Weston, L.A. Effects of UV-B on secondary metabolites of St. John’s Wort (Hypericum perforatum L.) grown in controlled environments. Photochem. Photobiol 2011, 87, 680–684. [Google Scholar]
- Rowe, L.D.; Norman, J.O.; Corrier, D.E.; Casteel, S.W.; Rector, B.S.; Bailey, E.M.; Schuster, J.L.; Reagor, J.C. Photosensitization of cattle in southeast Texas: Identification of phototoxic activity associated with Cooperia pedunculata. Am. J. Vet. Res 1987, 48, 1658–1661. [Google Scholar]
- Weston, L.A.; Ryan, P.R.; Watt, M. Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J. Exp. Bot 2012, 63, 3445–3454. [Google Scholar]
- Montiero-Riveiere, N. The Integument. Dellman’s Textbook of Veterinary Histology, 6th ed; Blackwell Press: Ames, IA, USA, 2006. [Google Scholar]
- Campbell, W.M.; Dombroski, G.S.; Sharma, I.; Partridge, A.C.; Collett, M.G. Photodynamic chlorophyll a metabolites, including phytoporphyrin (phylloerythrin), in the blood of photosensitive livestock: Overview and measurement. N. Z. Vet. J 2010, 58, 146–154. [Google Scholar]
- Keeler, R.F.; Tu, A.T. Plant and Fungal Toxins. In Handbook of Natural Toxins; Marcel Dekker: New York, NY, USA, 1983; Volume 1. [Google Scholar]
- Gupta, V.; Su, Y.S.; Wang, W.; Kardosh, A.; Liebes, L.F.; Hofman, F.M.; Schonthal, A.H.; Chen, T.C. Enhancement of glioblastoma cell killing by combination treatment with temozolomide and tamoxifen or hypericin. Neurosurg. Focus 2006, 20, E20. [Google Scholar]
- Monteiro-Riviere, N.A.; Inman, A.O.; Mak, V.; Wertz, P.; Riviere, J.E. Effect of selective lipid extraction from different body regions on epidermal barrier function. Pharmaceut. Res 2001, 18, 992–998. [Google Scholar]
- Stegelmeier, B.L. Equine photosensitization. Clin. Techn. Equine Pract 2002, 1, 81–88. [Google Scholar]
- Bourke, C.A. The effect of shade, shearing and wool type in the protection of Merino sheep from Hypericum perforatum (St. John’s wort) poisoning. Aust. Vet. J 2003, 81, 494–498. [Google Scholar]
- Bourke, C.A.; White, J.G. Reassessment of the toxicity of Hypericum perforatum (St. John’s wort) for cattle. Aust. Vet. J 2004, 82, 707–710. [Google Scholar]
- Monteiro-Riviere, N.A.; Bristol, D.G.; Manning, T.O.; Rogers, R.A.; Riviere, J.E. Interspecies and interregional analysis of the comparative histologic thickness and laser doppler blood-flow measurements at 5 cutaneous sites in 9 species. J. Invest. Dermatol 1990, 95, 582–586. [Google Scholar]
- Lagey, K.; Duinslaeger, L.; Vanderkelen, A. Burns induced by plants. Burns 1995, 21, 542–543. [Google Scholar]
- Mathieu, A.; Jeanmougin, M.; Cavelierballoy, B.; Ribrioux, A.; Civatte, J. Phytodermatitis with Heracleum-Mantegazzianum. Ann. Dermatol. Vener 1986, 113, 1005–1007. [Google Scholar]
- Lehmann, P. Sun exposed skin disease. Clin. Dermatol 2011, 29, 180–188. [Google Scholar]
- Tiley, G.E.D.; Dodd, F.S.; Wade, P.M. Heracleum mantegazzianum Sommier & Levier. J. Ecol 1996, 84, 297–319. [Google Scholar]
- Camm, E.; Buck, H.W.; Mitchell, J.C. Phytophotodermatitis from Heracleum mantegazzianum. Contact Dermat 1976, 2, 68–72. [Google Scholar]
- Musajo, L.; Rodighiero, G. Skin-photosensitizing furocoumarins. Experientia 1962, 18, 153. [Google Scholar]
- Knudsen, E.A. Seasonal variations in the content of phototoxic compounds in giant hogweed. Contact Dermat 1983, 9, 281–284. [Google Scholar]
- Berenbaum, M. Patterns of Furanocoumarin distribution and insect herbivory in the umbelliferae—Plant chemistry and community structure. Ecology 1981, 62, 1254–1266. [Google Scholar]
- Sardari, S.; Mori, Y.; Horita, K.; Micetich, R.G.; Nishibe, S.; Daneshtalab, M. Synthesis and antifungal activity of coumarins and angular furanocoumarins. Bioorgan. Med. Chem 1999, 7, 1933–1940. [Google Scholar]
- Musajo, L.; Rodighie, G.; Breccia, A.; Dallacqu, F.; Malesani, G. Photoreaction between DNA and skin-photosensitizing furocoumarins studied using labelled bergapten. Experientia 1966, 22, 75. [Google Scholar]
- Kitamura, N.; Kohtani, S.; Nakagaki, R. Molecular aspects of furocoumarin reactions: Photophysics, photochemistry, photobiology, and structural analysis. J. Photochem. Photobiol 2005, 6, 168–185. [Google Scholar]
- Bissonnette, L.; Arnason, J.T.; Smith, M.L. Real-time fluorescence-based detection of furanocoumarin photoadducts of DNA. Phytochem. Anal 2008, 19, 342–347. [Google Scholar]
- Bordin, F.; Carlassare, F.; Baccichetti, F.; Anselmo, L. DNA repair and recovery in Escherichia coli after psoralen and angelicin photosensitization. Biochim. Biophys. Acta 1976, 447, 249–259. [Google Scholar]
- Rodighiero, P.; Guiotto, A.; Chilin, A.; Bordin, F.; Baccichetti, F.; Carlassare, F.; Vedaldi, D.; Caffieri, S.; Pozzan, A.; Dall’Acqua, F. Angular furoquinolinones, psoralen analogs: Novel antiproliferative agents for skin diseases. Synthesis, biological activity, mechanism of action, and computer-aided studies. J. Med. Chem 1996, 39, 1293–1302. [Google Scholar]
- Amici, L.A.; Gasparro, F.P. 5-Methoxypsoralen photoadduct formation: Conversion of monoadducts to crosslinks. Photodermatol. Photoimmunol. Photomed 1995, 11, 135–139. [Google Scholar]
- Cole, R.S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim. Biophys. Acta 1971, 254, 30–39. [Google Scholar]
- Andrews, A.H.; Giles, C.J.; Thomsett, L.R. Suspected poisoning of a goat by Giant Hogweed. Vet. Rec 1985, 116, 205–207. [Google Scholar]
- Hintermann, J. Dermatosis in a dog due to Heracleum Montegazzianum Somm. and Levier. Schweiz. Arch. Tierheilkd 1967, 109, 654–656. [Google Scholar]
- Harwood, D.G. Giant hogweed and ducklings. Vet. Record 1985, 116, 300. [Google Scholar]
- Dolowy, W.C. Giant hogweed photodermatitis in two dogs in Bellevue, Washington. J. Am. Vet. Med. Assoc 1996, 209, 722. [Google Scholar]
- Pace, N.; Mackinney, G. Hypericin, the photodynamic pigment from St. John’s wort. J. Am. Chem. Soc 1941, 63, 2570–2574. [Google Scholar]
- Cunningham, I.J. Photosensitivity disease in New Zealand. N. Z. J. Sci. Technol 1947, 29, 207–213. [Google Scholar]
- Bourke, C.A. Sunlight associated hyperthermia as a consistent and rapidly developing clinical sign in sheep intoxicated by St. John’s wort (Hypericum perforatum). Aust. Vet. J 2000, 78, 483–488. [Google Scholar]
- Araya, O.S.; Ford, E.J. An investigation of the type of photosensitization caused by the ingestion of St. John’s Wort (Hypericum perforatum) by calves. J. Comp. Pathol 1981, 91, 135–141. [Google Scholar]
- Holzl, J.; Ostrowski, E. Analysis of the essential compounds of hypericum perforatum. Planta Med 1986, 531–531. [Google Scholar]
- Bourke, C.A.; Rayward, D. Photosensitisation in dairy cattle grazing alligator weed (Alternanthera philoxeroides) infested pastures. Aust. Vet. J 2003, 81, 361–362. [Google Scholar]
- Agostinis, P.; Vantieghem, A.; Merlevede, W.; de Witte, P.A. Hypericin in cancer treatment: More light on the way. Int. J. Biochem. Cell Biol 2002, 34, 221–241. [Google Scholar]
- Chen, B.; Xu, Y.; Roskams, T.; Delaey, E.; Agostinis, P.; Vandenheede, J.R.; de Witte, P. Efficacy of antitumoral photodynamic therapy with hypericin: relationship between biodistribution and photodynamic effects in the RIF-1 mouse tumor model. Int. J. Cancer 2001, 93, 275–282. [Google Scholar]
- Diwu, Z.; Lown, J.W. Photosensitization with anticancer agents. 17. EPR studies of photodynamic action of hypericin: formation of semiquinone radical and activated oxygen species on illumination. Free Radic. Biol. Med 1993, 14, 209–215. [Google Scholar]
- Roslaniec, M.; Weitman, H.; Freeman, D.; Mazur, Y.; Ehrenberg, B. Liposome binding constants and singlet oxygen quantum yields of hypericin, tetrahydroxy helianthrone and their derivatives: Studies in organic solutions and in liposomes. J. Photochem. Photobiol. B 2000, 57, 149–158. [Google Scholar]
- Ali, S.M.; Chee, S.K.; Yuen, G.Y.; Olivo, M. Hypocrellins and Hypericin induced apoptosis in human tumor cells: A possible role of hydrogen peroxide. Int. J. Mol. Med 2002, 9, 461–472. [Google Scholar]
- Vantieghem, A.; Assefa, Z.; Vandenabeele, P.; Declercq, W.; Courtois, S.; Vandenheede, J.R.; Merlevede, W.; de Witte, P.; Agostinis, P. Hypericin-induced photosensitization of HeLa cells leads to apoptosis or necrosis. Involvement of cytochrome c and procaspase-3 activation in the mechanism of apoptosis. FEBS Lett 1998, 440, 19–24. [Google Scholar]
- Assefa, Z.; Vantieghem, A.; Declercq, W.; Vandenabeele, P.; Vandenheede, J.R.; Merlevede, W.; de Witte, P.; Agostinis, P. The activation of the c-Jun N-terminal kinase and p38 mitogen-activated protein kinase signaling pathways protects HeLa cells from apoptosis following photodynamic therapy with hypericin. J. Biol. Chem 1999, 274, 8788–8796. [Google Scholar]
- Semelakova, M.; Mikes, J.; Jendzelovsky, R.; Fedorocko, P. The pro-apoptotic and anti-invasive effects of hypericin-mediated photodynamic therapy are enhanced by hyperforin or aristoforin in HT-29 colon adenocarcinoma cells. J. Photochem. Photobiol. B 2012, 117, 115–125. [Google Scholar]
- Buytaert, E.; Callewaert, G.; Hendrickx, N.; Scorrano, L.; Hartmann, D.; Missiaen, L.; Vandenheede, J.R.; Heirman, I.; Grooten, J.; Agostinis, P. Role of endoplasmic reticulum depletion and multidomain proapoptotic BAX and BAK proteins in shaping cell death after hypericin-mediated photodynamic therapy. FASEB J 2006, 20, 756–758. [Google Scholar]
- Ali, S.M.; Chee, S.K.; Yuen, G.Y.; Olivo, M. Hypericin induced death receptor-mediated apoptosis in photoactivated tumor cells. Int. J. Mol. Med 2002, 9, 601–616. [Google Scholar]
- Ali, S.M.; Olivo, M. Bio-distribution and subcellular localization of Hypericin and its role in PDT induced apoptosis in cancer cells. Int. J. Oncol 2002, 21, 531–540. [Google Scholar]
- Ritz, R.; Roser, F.; Radomski, N.; Strauss, W.S.; Tatagiba, M.; Gharabaghi, A. Subcellular colocalization of hypericin with respect to endoplasmic reticulum and Golgi apparatus in glioblastoma cells. Anticancer Res 2008, 28, 2033–2038. [Google Scholar]
- Huntosova, V.; Nadova, Z.; Dzurova, L.; Jakusova, V.; Sureau, F.; Miskovsky, P. Cell death response of U87 glioma cells on hypericin photoactivation is mediated by dynamics of hypericin subcellular distribution and its aggregation in cellular organelles. Photochem. Photobiol. Sci 2012, 11, 1428–1436. [Google Scholar]
- Ho, Y.F.; Wu, M.H.; Cheng, B.H.; Chen, Y.W.; Shih, M.C. Lipid-mediated preferential localization of hypericin in lipid membranes. Biochim. Biophys. Acta 2009, 1788, 1287–1295. [Google Scholar]
- Chaloupka, R.; Obsil, T.; Plasek, J.; Sureau, F. The effect of hypericin and hypocrellin-A on lipid membranes and membrane potential of 3T3 fibroblasts. Biochim. Biophys. Acta 1999, 1418, 39–47. [Google Scholar]
- Scheie, E.; Smith, B.L.; Cox, N.; Flaoyen, A. Spectrofluorometric analysis of phylloerythrin (phytoporphyrin) in plasma and tissues from sheep suffering from facial eczema. N. Z. Vet. J 2003, 51, 104–110. [Google Scholar]
- Scheie, E.; Flaoyen, A.; Moan, J.; Berg, K. Phylloerythrin: Mechanisms for cellular uptake and location, photosensitisation and spectroscopic evaluation. N. Z. Vet. J 2002, 50, 104–110. [Google Scholar]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herbeger, J.P. World’s Worst Weeds. Distribution and Biology; University of Hawaii: Honolulu, HI, USA, 1977. [Google Scholar]
- Sharma, O.P.; Sharma, S.; Pattabhi, V.; Mahato, S.B.; Sharma, P.D. A review of the hepatotoxic plant Lantana camara. Crit. Rev. Toxicol 2007, 37, 313–352. [Google Scholar]
- Sharma, O.P.; Dawra, R.K.; Pattabhi, V. Molecular-structure, polymorphism, and toxicity of lantadene-a, the pentacyclic triterpenoid from the hepatotoxic plant Lantana-camara. J. Biochem. Toxicol 1991, 6, 57–63. [Google Scholar]
- Sharma, O.P.; Vaid, J.; Pattabhi, V.; Bhutani, K.K. Biological action of lantadene-C, a new hepatotoxicant from Lantana-camara var aculeata. J. Biochem. Toxicol 1992, 7, 73–79. [Google Scholar]
- Pass, M.A. Current ideas on the pathophysiology and treatment of lantana poisoning of ruminants. Aust. Vet. J 1986, 63, 169–171. [Google Scholar]
- Pass, M.A.; McSweeney, C.S.; Reynoldson, J.A. Absorption of the toxins of Lantana camara L. from the digestive system of sheep. J. Appl. Toxicol 1981, 1, 38–41. [Google Scholar]
- Pass, M.A.; Heath, T.J. Effect of Lantana camara on intestinal motility in sheep. J. Comp. Pathol 1978, 88, 149–156. [Google Scholar]
- Lee, S.T.; Mitchell, R.B.; Wang, Z.R.; Heiss, C.; Gardner, D.R.; Azadi, P. Isolation, characterization, and quantification of steroidal saponins in Switchgrass (Panicum virgatum L.). J. Agric. Food Chem 2009, 57, 2599–2604. [Google Scholar]
- Puoli, J.R.; Reid, R.L.; Belesky, D.P. Photosensitization in lambs grazing Switchgrass. Agron. J 1992, 84, 1077–1080. [Google Scholar]
- Everist, E. Poisonous Plants of Australia; Angus and Robertson: Sydney, Australia, 1974. [Google Scholar]
- Smith, L.W.; Culvenor, C.C.J. Plant sources of hepatotoxic pyrrolizidine alkaloids. J. Nat. Prod 1981, 44, 129–152. [Google Scholar]
- Stegelmeier, B.L. Pyrrolizidine alkaloid-containing toxic plants (Senecio, Crotalaria, Cynoglossum, Amsinckia, Heliotropium, and Echium spp.). Vet. Clin. N. Am. Food A 2011, 27, 419–428. [Google Scholar]
- Stegelmeier, B.L.; Edgar, J.A.; Colegate, S.M.; Gardner, D.R.; Schoch, T.K.; Coulombe, R.A.; Molyneux, R.J. Pyrrolizidine alkaloid plants, metabolism and toxicity. J. Nat. Toxins 1999, 8, 95–116. [Google Scholar]
- Adams, R. Veterinary Pharmacology and Therapeutics, 8th ed; Iowa State University Press: Ames, IA, USA, 2001. [Google Scholar]
- Mattocks, A.R. Chemistry and Toxicology of Pyrrolizidine Alkaloids; Academic Press: Orlando, FL, USA, 1986. [Google Scholar]
- Cheeke, P.R. Toxicity and metabolism of pyrrolizidine alkaloids. J. Anim. Sci 1988, 66, 2343–2350. [Google Scholar]
- Cheeke, P.R. Comparative toxicity and metabolism of pyrrolizidine alkaloids in ruminants and nonruminant herbivores. Can. J. Anim. Sci 1984, 64, 201–202. [Google Scholar]
- Culvenor, C.C.J. The alkaloids of Echium-plantagineum L. 1. Echiumine and echimidine. Aust. J. Chem 1956, 9, 512–520. [Google Scholar]
- Ghamkhar, K.; Revell, C.; Erskine, W. Biserrula pelecinus L.—Genetic diversity in a promising pasture legume for the future. Crop Pasture Sci 2012, 63, 833–839. [Google Scholar]
- Howieson, J.G.; Loi, A.; Carr, S.J. Biserrula-Pelecinus L.—A legume pasture species with potential for acid, duplex soils which is nodulated by unique root-nodule bacteria. Aust. J. Agric. Res 1995, 46, 997–1009. [Google Scholar]
- Loi, A.; Cocks, P.S.; Howieson, J.G.; Carr, S.J. Morphological characterization of Mediterranean populations of Biserrula pelecinus L. Plant Breed 1997, 116, 171–176. [Google Scholar]
- Loi, A.; Nutt, B.J.; Revell, C.K.; Sandral, G.A.; Dear, B.S. Mauro: A mid to late maturing cultivar of biserrula (Biserrula pelecinus). Aust. J. Exp. Agric 2006, 46, 595–597. [Google Scholar]
- Hackney, B.; Rodham, C.; Piltz, J. Using Biserrula to Increase Crop and Livestock Production. In Meat and Livestock Australia; NSW Department of Primary Industries: Orange, NSW, Australia, 2012. [Google Scholar]
- Quinn, J.A.; Lardner, G.; Kessell, A.; Weston, L.A. Photosensitization and its Epidemiology in Grazing Sheep in Southeastern AUSTRALIA as Caused by Biserrula pelecinus. In Graham Centre Bulletin; Graham Centre for Agricultural Innovation: Wagga Wagga, Australia, 2013; in preparation. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Quinn, J.C.; Kessell, A.; Weston, L.A. Secondary Plant Products Causing Photosensitization in Grazing Herbivores: Their Structure, Activity and Regulation. Int. J. Mol. Sci. 2014, 15, 1441-1465. https://doi.org/10.3390/ijms15011441
Quinn JC, Kessell A, Weston LA. Secondary Plant Products Causing Photosensitization in Grazing Herbivores: Their Structure, Activity and Regulation. International Journal of Molecular Sciences. 2014; 15(1):1441-1465. https://doi.org/10.3390/ijms15011441
Chicago/Turabian StyleQuinn, Jane C., Allan Kessell, and Leslie A. Weston. 2014. "Secondary Plant Products Causing Photosensitization in Grazing Herbivores: Their Structure, Activity and Regulation" International Journal of Molecular Sciences 15, no. 1: 1441-1465. https://doi.org/10.3390/ijms15011441




