Apoptosis Signal Regulating Kinase 1 (ASK1): Potential as a Therapeutic Target for Alzheimer’s Disease
Abstract
:1. Introduction
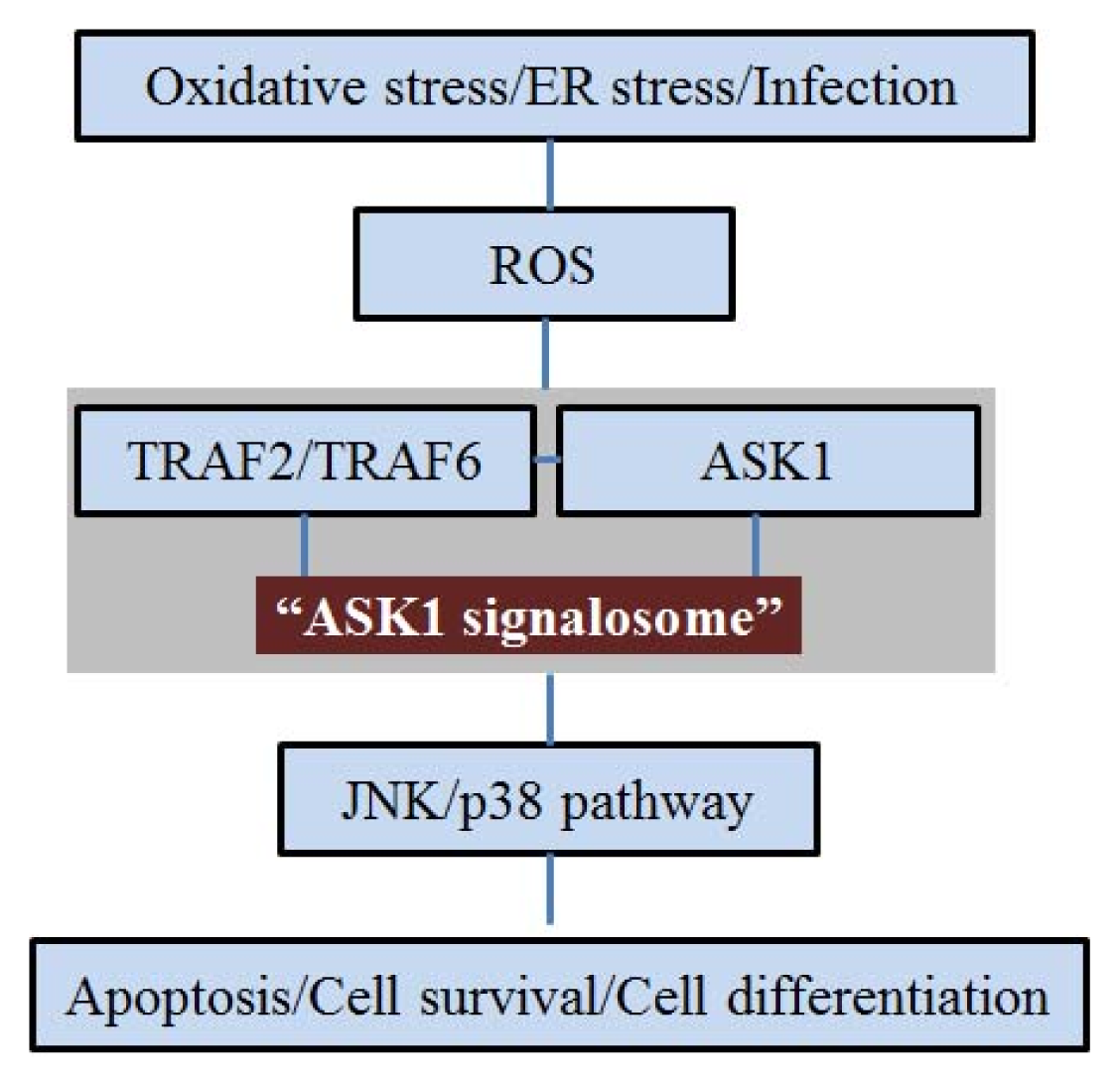
2. ASK1 and Oxidative Stress
3. ASK1 and ER Stress
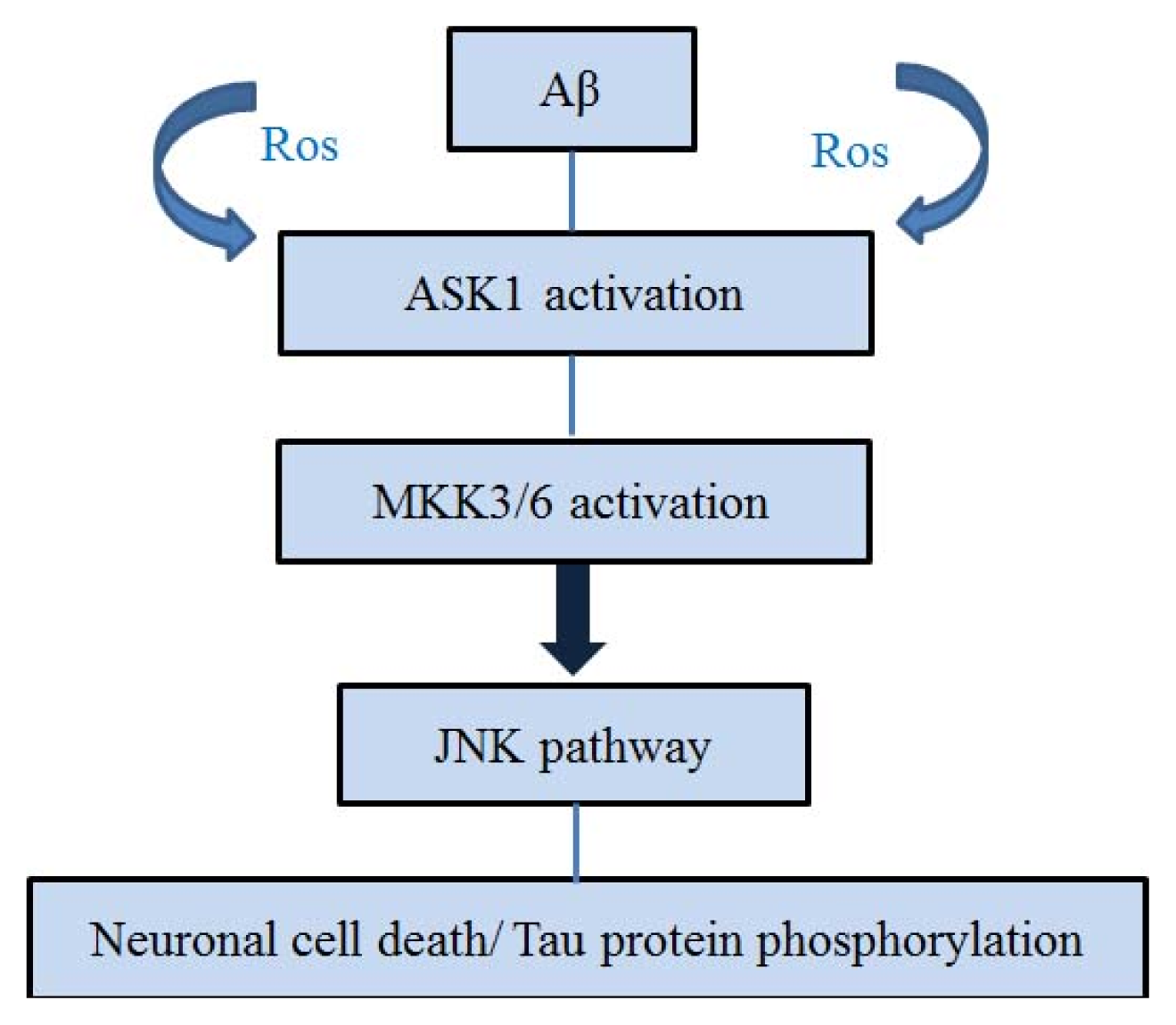
4. ASK1 and Aβ
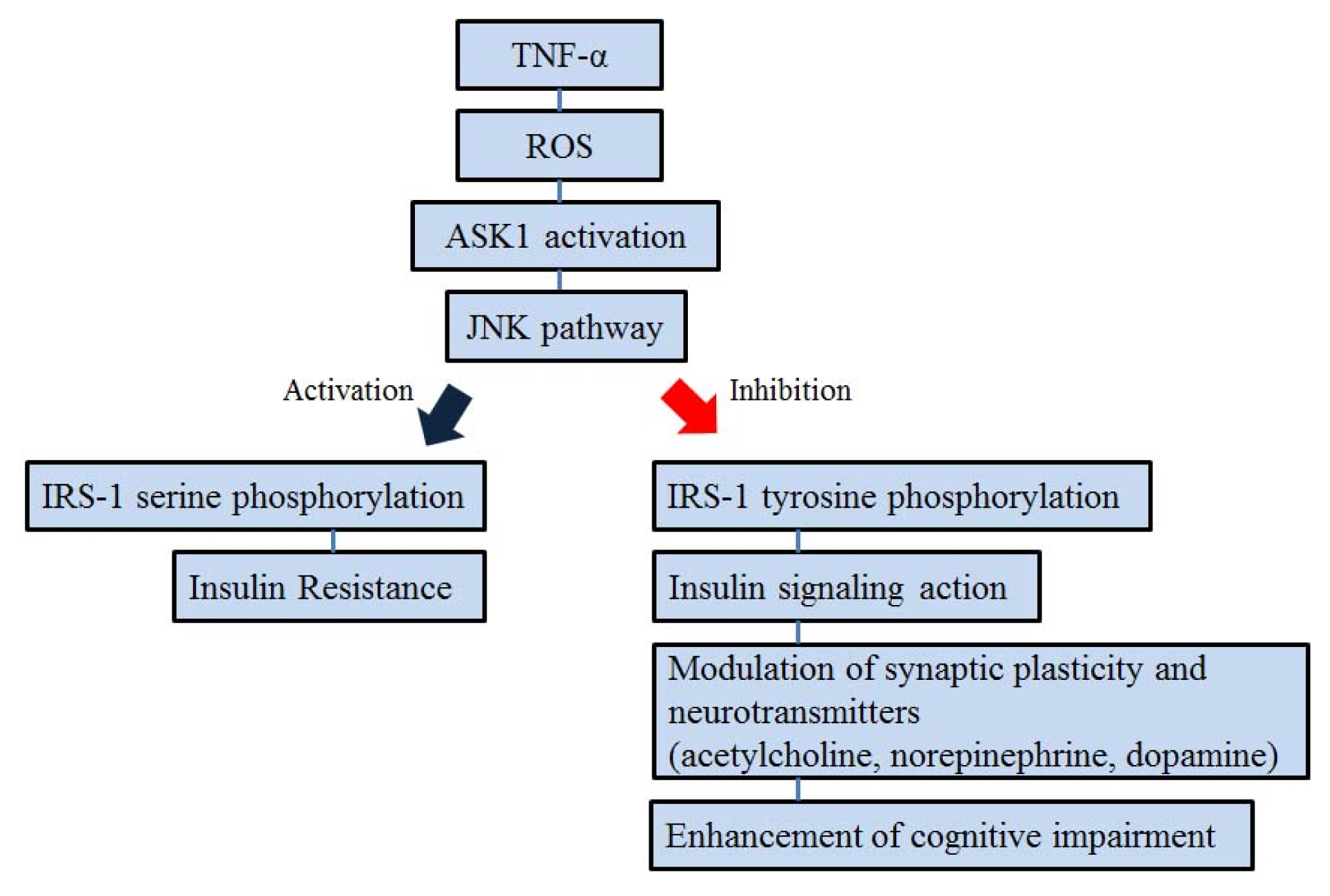
5. ASK1 and Insulin Signal Transduction
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hyman, B.T.; Damasio, H.; Damasio, A.R.; van Hoesen, G.W. Alzheimer’s disease. Annu. Rev. Public Health 1989, 10, 115–140. [Google Scholar]
- Van Hoesen, G.W.; Augustinack, J.C.; Dierking, J.; Redman, S.J.; Thangavel, R. The parahippocampal gyrus in Alzheimer’s disease. Clinical and preclinical neuroanatomical correlates. Ann. N. Y. Acad. Sci 2000, 911, 254–274. [Google Scholar]
- Ebenezer, P.J.; Weidner, A.M.; LeVine, H., 3rd; Markesbery, W.R.; Murphy, M.P.; Zhang, L.; Dasuri, K.; Fernandez-Kim, S.O.; Bruce-Keller, A.J.; Gavilan, E.; et al. Neuron specific toxicity of oligomeric amyloid-beta: Role for JUN-kinase and oxidative stress. J. Alzheimer’s Dis 2010, 22, 839–848. [Google Scholar]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 1998, 17, 2596–2606. [Google Scholar]
- Tobiume, K.; Matsuzawa, A.; Takahashi, T.; Nishitoh, H.; Morita, K.; Takeda, K.; Minowa, O.; Miyazono, K.; Noda, T.; Ichijo, H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2001, 2, 222–228. [Google Scholar]
- Takeda, K.; Hatai, T.; Hamazaki, T.S.; Nishitoh, H.; Saitoh, M.; Ichijo, H. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J. Biol. Chem 2000, 275, 9805–9813. [Google Scholar]
- Sayama, K.; Hanakawa, Y.; Shirakata, Y.; Yamasaki, K.; Sawada, Y.; Sun, L.; Yamanishi, K.; Ichijo, H.; Hashimoto, K. Apoptosis signal-regulating kinase 1 (ASK1) is an intracellular inducer of keratinocyte differentiation. J. Biol. Chem 2001, 276, 999–1004. [Google Scholar]
- Hwang, J.R.; Zhang, C.; Patterson, C. C-terminus of heat shock protein 70-interacting protein facilitates degradation of apoptosis signal-regulating kinase 1 and inhibits apoptosis signal-regulating kinase 1-dependent apoptosis. Cell Stress Chaperones 2005, 10, 147–156. [Google Scholar]
- Goldman, E.H.; Chen, L.; Fu, H. Activation of apoptosis signal-regulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J. Biol. Chem 2004, 279, 10442–10449. [Google Scholar]
- Ouyang, M.; Shen, X. Critical role of ASK1 in the 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Neurochem 2006, 97, 234–244. [Google Scholar]
- Jucker, M.; Walker, L.C. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol 2011, 70, 532–540. [Google Scholar]
- Kadowaki, H.; Nishitoh, H.; Urano, F.; Sadamitsu, C.; Matsuzawa, A.; Takeda, K.; Masutani, H.; Yodoi, J.; Urano, Y.; Nagano, T.; et al. Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ 2005, 12, 19–24. [Google Scholar]
- Nakagawa, T.; Zhu, H.; Morishima, N.; Li, E.; Xu, J.; Yankner, B.A.; Yuan, J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 2000, 403, 98–103. [Google Scholar]
- Imaizumi, K.; Miyoshi, K.; Katayama, T.; Yoneda, T.; Taniguchi, M.; Kudo, T.; Tohyama, M. The unfolded protein response and Alzheimer’s disease. Biochim. Biophys. Acta 2001, 1536, 85–96. [Google Scholar]
- Nishitoh, H.; Matsuzawa, A.; Tobiume, K.; Saegusa, K.; Takeda, K.; Inoue, K.; Hori, S.; Kakizuka, A.; Ichijo, H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 2002, 16, 1345–1355. [Google Scholar]
- Hueber, A.O.; Zornig, M.; Lyon, D.; Suda, T.; Nagata, S.; Evan, G.I. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science 1997, 278, 1305–1309. [Google Scholar]
- Wei, W.; Norton, D.D.; Wang, X.; Kusiak, J.W. Abeta 17–42 in Alzheimer’s disease activates JNK and caspase-8 leading to neuronal apoptosis. Brain: J. Neurol 2002, 125, 2036–2043. [Google Scholar]
- Peel, A.L.; Sorscher, N.; Kim, J.Y.; Galvan, V.; Chen, S.; Bredesen, D.E. Tau phosphorylation in Alzheimer’s disease: Potential involvement of an APP-MAP kinase complex. Neuromol. Med 2004, 5, 205–218. [Google Scholar]
- Hoyer, S. Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J. Neural Transm 1998, 105, 415–422. [Google Scholar]
- Hoyer, S. The aging brain. Changes in the neuronal insulin/insulin receptor signal transduction cascade trigger late-onset sporadic Alzheimer disease (SAD). A mini-review. J. Neural Transm 2002, 109, 991–1002. [Google Scholar]
- Frolich, L.; Blum-Degen, D.; Riederer, P.; Hoyer, S. A disturbance in the neuronal insulin receptor signal transduction in sporadic Alzheimer’s disease. Ann. N. Y. Acad. Sci 1999, 893, 290–293. [Google Scholar]
- Hoyer, S.; Lannert, H. Inhibition of the neuronal insulin receptor causes Alzheimer-like disturbances in oxidative/energy brain metabolism and in behavior in adult rats. Ann. N. Y. Acad. Sci 1999, 893, 301–303. [Google Scholar]
- Watson, G.S.; Craft, S. The role of insulin resistance in the pathogenesis of Alzheimer’s disease: Implications for treatment. CNS Drugs 2003, 17, 27–45. [Google Scholar]
- Cholerton, B.; Baker, L.D.; Craft, S. Insulin, cognition, and dementia. Eur. J. Pharmacol 2013. [Google Scholar] [CrossRef]
- Nishikawa, T.; Kukidome, D.; Sonoda, K.; Fujisawa, K.; Matsuhisa, T.; Motoshima, H.; Matsumura, T.; Araki, E. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res. Clin. Pract 2007, 77, S161–S164. [Google Scholar]
- Ichijo, H.; Nishida, E.; Irie, K.; ten Dijke, P.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar]
- Hsieh, C.C.; Papaconstantinou, J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J 2006, 20, 259–268. [Google Scholar]
- Matsuzawa, A.; Nishitoh, H.; Tobiume, K.; Takeda, K.; Ichijo, H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: Advanced findings from ASK1 knockout mice. Antioxid. Redox Signal 2002, 4, 415–425. [Google Scholar]
- Chen, J.T.; Fong, Y.C.; Li, T.M.; Liu, J.F.; Hsu, C.W.; Chang, C.S.; Tang, C.H. DDTD, an isoflavone derivative, induces cell apoptosis through the reactive oxygen species/apoptosis signal-regulating kinase 1 pathway in human osteosarcoma cells. Eur. J. Pharmacol 2008, 597, 19–26. [Google Scholar]
- Zhang, L.; Chen, J.; Fu, H. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc. Natl. Acad. Sci. USA 1999, 96, 8511–8515. [Google Scholar]
- Lau, J.M.; Jin, X.; Ren, J.; Avery, J.; DeBosch, B.J.; Treskov, I.; Lupu, T.S.; Kovacs, A.; Weinheimer, C.; Muslin, A.J. The 14-3-3 tau phosphoserine-binding protein is required for cardiomyocyte survival. Mol. Cell. Biol 2007, 27, 1455–1466. [Google Scholar]
- Gotoh, Y.; Cooper, J.A. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J. Biol. Chem 1998, 273, 17477–17482. [Google Scholar]
- Matsuzawa, A.; Saegusa, K.; Noguchi, T.; Sadamitsu, C.; Nishitoh, H.; Nagai, S.; Koyasu, S.; Matsumoto, K.; Takeda, K.; Ichijo, H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol 2005, 6, 587–592. [Google Scholar]
- Harding, H.P.; Ron, D. Endoplasmic reticulum stress and the development of diabetes: A review. Diabetes 2002, 51, S455–S461. [Google Scholar]
- Rutkowski, D.T.; Kaufman, R.J. A trip to the ER: Coping with stress. Trends Cell Biol 2004, 14, 20–28. [Google Scholar]
- Katayama, T.; Imaizumi, K.; Sato, N.; Miyoshi, K.; Kudo, T.; Hitomi, J.; Morihara, T.; Yoneda, T.; Gomi, F.; Mori, Y.; et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat. Cell Biol 1999, 1, 479–485. [Google Scholar]
- Sato, N.; Imaizumi, K.; Manabe, T.; Taniguchi, M.; Hitomi, J.; Katayama, T.; Yoneda, T.; Morihara, T.; Yasuda, Y.; Takagi, T.; et al. Increased production of beta-amyloid and vulnerability to endoplasmic reticulum stress by an aberrant spliced form of presenilin 2. J. Biol. Chem 2001, 276, 2108–2114. [Google Scholar]
- Katayama, T.; Imaizumi, K.; Honda, A.; Yoneda, T.; Kudo, T.; Takeda, M.; Mori, K.; Rozmahel, R.; Fraser, P.; George-Hyslop, P.S.; et al. Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer’s disease-linked presenilin-1 mutations. J. Biol. Chem 2001, 276, 43446–43454. [Google Scholar]
- Guo, Q.; Sebastian, L.; Sopher, B.L.; Miller, M.W.; Ware, C.B.; Martin, G.M.; Mattson, M.P. Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid beta-peptide toxicity: Central roles of superoxide production and caspase activation. J. Neurochem 1999, 72, 1019–1029. [Google Scholar]
- Guo, Q.; Fu, W.; Sopher, B.L.; Miller, M.W.; Ware, C.B.; Martin, G.M.; Mattson, M.P. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat. Med 1999, 5, 101–106. [Google Scholar]
- Nishitoh, H.; Saitoh, M.; Mochida, Y.; Takeda, K.; Nakano, H.; Rothe, M.; Miyazono, K.; Ichijo, H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell 1998, 2, 389–395. [Google Scholar]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666. [Google Scholar]
- Johnson, G.A.; Spencer, T.E.; Hansen, T.R.; Austin, K.J.; Burghardt, R.C.; Bazer, F.W. Expression of the interferon tau inducible ubiquitin cross-reactive protein in the ovine uterus. Biol. Reprod 1999, 61, 312–318. [Google Scholar]
- Selkoe, D.J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu. Rev. Neurosci 1994, 17, 489–517. [Google Scholar]
- Selkoe, D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J. Alzheimer’s Dis 2001, 3, 75–80. [Google Scholar]
- Kang, J.; Lemaire, H.G.; Unterbeck, A.; Salbaum, J.M.; Masters, C.L.; Grzeschik, K.H.; Multhaup, G.; Beyreuther, K.; Muller-Hill, B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 1987, 325, 733–736. [Google Scholar]
- Selkoe, D.J. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature 1999, 399, A23–A31. [Google Scholar]
- Yankner, B.A. Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron 1996, 16, 921–932. [Google Scholar]
- Hsu, M.J.; Hsu, C.Y.; Chen, B.C.; Chen, M.C.; Ou, G.; Lin, C.H. Apoptosis signal-regulating kinase 1 in amyloid beta peptide-induced cerebral endothelial cell apoptosis. J. Neurosci 2007, 27, 5719–5729. [Google Scholar]
- Farias, G.; Cornejo, A.; Jimenez, J.; Guzman, L.; Maccioni, R.B. Mechanisms of tau self-aggregation and neurotoxicity. Curr. Alzheimer Res 2011, 8, 608–614. [Google Scholar]
- Johnson, G.V.; Stoothoff, W.H. Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci 2004, 117, 5721–5729. [Google Scholar]
- Morishima, Y.; Gotoh, Y.; Zieg, J.; Barrett, T.; Takano, H.; Flavell, R.; Davis, R.J.; Shirasaki, Y.; Greenberg, M.E. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci 2001, 21, 7551–7560. [Google Scholar]
- Song, S.; Kim, S.Y.; Hong, Y.M.; Jo, D.G.; Lee, J.Y.; Shim, S.M.; Chung, C.W.; Seo, S.J.; Yoo, Y.J.; Koh, J.Y.; et al. Essential role of E2-25K/Hip-2 in mediating amyloid-beta neurotoxicity. Mol. Cell 2003, 12, 553–563. [Google Scholar]
- Kihiko, M.E.; Tucker, H.M.; Rydel, R.E.; Estus, S. c-Jun contributes to amyloid beta-induced neuronal apoptosis but is not necessary for amyloid beta-induced c-jun induction. J. Neurochem 1999, 73, 2609–2612. [Google Scholar]
- Reynolds, C.H.; Betts, J.C.; Blackstock, W.P.; Nebreda, A.R.; Anderton, B.H. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: Differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J. Neurochem 2000, 74, 1587–1595. [Google Scholar]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev 2001, 81, 807–869. [Google Scholar]
- Hashimoto, Y.; Niikura, T.; Chiba, T.; Tsukamoto, E.; Kadowaki, H.; Nishitoh, H.; Yamagishi, Y.; Ishizaka, M.; Yamada, M.; Nawa, M.; et al. The cytoplasmic domain of Alzheimer’s amyloid-beta protein precursor causes sustained apoptosis signal-regulating kinase 1/c-Jun NH2-terminal kinase-mediated neurotoxic signal via dimerization. J. Pharmacol. Exp. Ther 2003, 306, 889–902. [Google Scholar]
- Maruoka, S.; Hashimoto, S.; Gon, Y.; Nishitoh, H.; Takeshita, I.; Asai, Y.; Mizumura, K.; Shimizu, K.; Ichijo, H.; Horie, T. ASK1 regulates influenza virus infection-induced apoptotic cell death. Biochem. Biophys. Res. Commun 2003, 307, 870–876. [Google Scholar]
- Lu, D.C.; Shaked, G.M.; Masliah, E.; Bredesen, D.E.; Koo, E.H. Amyloid beta protein toxicity mediated by the formation of amyloid-beta protein precursor complexes. Ann. Neurol 2003, 54, 781–789. [Google Scholar]
- Galvan, V.; Banwait, S.; Spilman, P.; Gorostiza, O.F.; Peel, A.; Ataie, M.; Crippen, D.; Huang, W.; Sidhu, G.; Ichijo, H.; et al. Interaction of ASK1 and the beta-amyloid precursor protein in a stress-signaling complex. Neurobiol. Dis 2007, 28, 65–75. [Google Scholar]
- Akterin, S.; Cowburn, R.F.; Miranda-Vizuete, A.; Jimenez, A.; Bogdanovic, N.; Winblad, B.; Cedazo-Minguez, A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer’s disease. Cell Death Differ 2006, 13, 1454–1465. [Google Scholar]
- Craft, S.; Asthana, S.; Newcomer, J.W.; Wilkinson, C.W.; Matos, I.T.; Baker, L.D.; Cherrier, M.; Lofgreen, C.; Latendresse, S.; Petrova, A.; et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch. Gen. Psychiatry 1999, 56, 1135–1140. [Google Scholar]
- Kern, W.; Peters, A.; Fruehwald-Schultes, B.; Deininger, E.; Born, J.; Fehm, H.L. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology 2001, 74, 270–280. [Google Scholar]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar]
- Aguirre, V.; Werner, E.D.; Giraud, J.; Lee, Y.H.; Shoelson, S.E.; White, M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem 2002, 277, 1531–1537. [Google Scholar]
- Kern, W.; Born, J.; Schreiber, H.; Fehm, H.L. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes 1999, 48, 557–563. [Google Scholar]
- Park, C.R.; Seeley, R.J.; Craft, S.; Woods, S.C. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol. Behav 2000, 68, 509–514. [Google Scholar]
- Craft, S.; Asthana, S.; Schellenberg, G.; Cherrier, M.; Baker, L.D.; Newcomer, J.; Plymate, S.; Latendresse, S.; Petrova, A.; Raskind, M.; et al. Insulin metabolism in Alzheimer’s disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology 1999, 70, 146–152. [Google Scholar]
- Van der Heide, L.P.; Ramakers, G.M.; Smidt, M.P. Insulin signaling in the central nervous system: Learning to survive. Prog. Neurobiol 2006, 79, 205–221. [Google Scholar]
- Plum, L.; Schubert, M.; Bruning, J.C. The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab 2005, 16, 59–65. [Google Scholar]
- Cole, A.R.; Astell, A.; Green, C.; Sutherland, C. Molecular connexions between dementia and diabetes. Neurosci. Biobehav. Rev 2007, 31, 1046–1063. [Google Scholar]
- Dou, J.T.; Chen, M.; Dufour, F.; Alkon, D.L.; Zhao, W.Q. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn. Mem 2005, 12, 646–655. [Google Scholar]
- Figlewicz, D.P.; Szot, P.; Israel, P.A.; Payne, C.; Dorsa, D.M. Insulin reduces norepinephrine transporter mRNA in vivo in rat locus coeruleus. Brain Res 1993, 602, 161–164. [Google Scholar]
- Kopf, S.R.; Baratti, C.M. Effects of posttraining administration of insulin on retention of a habituation response in mice: Participation of a central cholinergic mechanism. Neurobiol. Learn. Mem 1999, 71, 50–61. [Google Scholar]
- Wang, Y.T.; Salter, M.W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature 1994, 369, 233–235. [Google Scholar]
- Wan, Q.; Xiong, Z.G.; Man, H.Y.; Ackerley, C.A.; Braunton, J.; Lu, W.Y.; Becker, L.E.; MacDonald, J.F.; Wang, Y.T. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature 1997, 388, 686–690. [Google Scholar]
- Van der Heide, L.P.; Kamal, A.; Artola, A.; Gispen, W.H.; Ramakers, G.M. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J. Neurochem 2005, 94, 1158–1166. [Google Scholar]
- Man, H.Y.; Lin, J.W.; Ju, W.H.; Ahmadian, G.; Liu, L.; Becker, L.E.; Sheng, M.; Wang, Y.T. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron 2000, 25, 649–662. [Google Scholar]
- Kneussel, M. Dynamic regulation of GABA(A) receptors at synaptic sites. Brain Res. Brain Res. Rev 2002, 39, 74–83. [Google Scholar]
- Malenka, R.C. Synaptic plasticity and AMPA receptor trafficking. Ann. N. Y. Acad. Sci 2003, 1003, 1–11. [Google Scholar]
- Huang, C.C.; Lee, C.C.; Hsu, K.S. An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus. J. Neurochem 2004, 89, 217–231. [Google Scholar]
- Araki, E.; Lipes, M.A.; Patti, M.E.; Bruning, J.C.; Haag, B., 3rd; Johnson, R.S.; Kahn, C.R. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 1994, 372, 186–190. [Google Scholar]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem 2000, 275, 9047–9054. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, J.; Park, K.A.; Lee, W.T.; Lee, J.E. Apoptosis Signal Regulating Kinase 1 (ASK1): Potential as a Therapeutic Target for Alzheimer’s Disease. Int. J. Mol. Sci. 2014, 15, 2119-2129. https://doi.org/10.3390/ijms15022119
Song J, Park KA, Lee WT, Lee JE. Apoptosis Signal Regulating Kinase 1 (ASK1): Potential as a Therapeutic Target for Alzheimer’s Disease. International Journal of Molecular Sciences. 2014; 15(2):2119-2129. https://doi.org/10.3390/ijms15022119
Chicago/Turabian StyleSong, Juhyun, Kyung Ah Park, Won Taek Lee, and Jong Eun Lee. 2014. "Apoptosis Signal Regulating Kinase 1 (ASK1): Potential as a Therapeutic Target for Alzheimer’s Disease" International Journal of Molecular Sciences 15, no. 2: 2119-2129. https://doi.org/10.3390/ijms15022119
APA StyleSong, J., Park, K. A., Lee, W. T., & Lee, J. E. (2014). Apoptosis Signal Regulating Kinase 1 (ASK1): Potential as a Therapeutic Target for Alzheimer’s Disease. International Journal of Molecular Sciences, 15(2), 2119-2129. https://doi.org/10.3390/ijms15022119




