Seed-Specific Expression of a Lysine-Rich Protein Gene, GhLRP, from Cotton Significantly Increases the Lysine Content in Maize Seeds
Abstract
:1. Introduction
2. Results
2.1. Gene Cloning and Plasmid Construction
2.2. Plant Transformation and Molecular Analyses
2.3. Lysine and Total Protein Contents in Transgenic Kernels
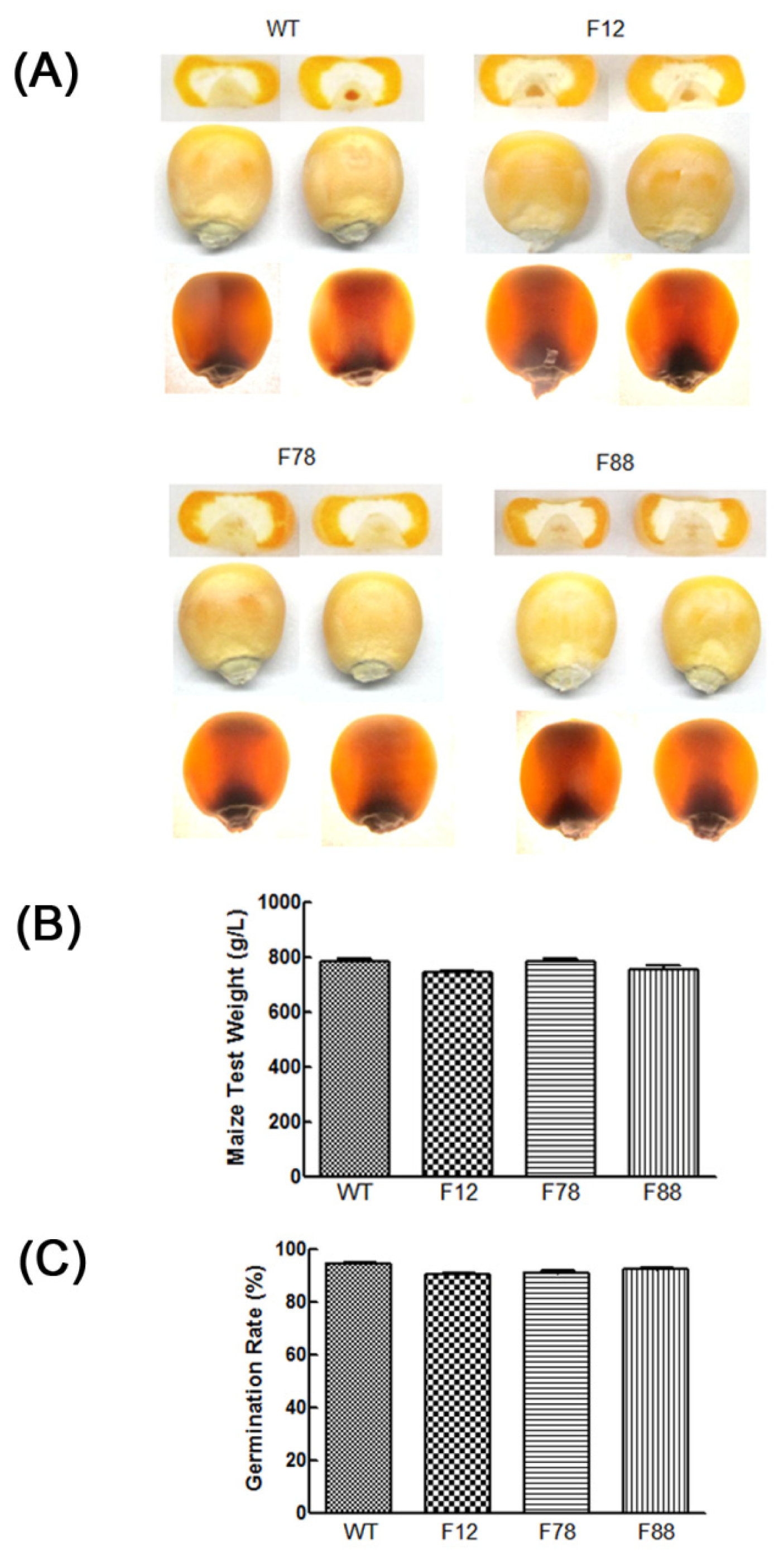
2.4. Starch and Lipid Contents in Transgenic Kernels
2.5. Zein and Non-Zein Proteins Accumulation in Transgenic Kernels
2.6. Agronomic Quality Analysis
3. Discussion
4. Experimental Section
4.1. Plant Materials and Growth Conditions
4.2. Plasmid Construction
4.3. Maize Transformation
4.4. PCR Analysis
4.5. RNA Extraction and RT-PCR and qRT-PCR Analyses
4.6. Antiserum Production, Protein Extraction and Western Blot Analysis
4.7. Lysine, Protein, Lipid and Starch Analyses
4.8. Zein and Non-Zein Proteins Extraction and Quantification
4.9. Agronomic Quality Measurement
4.10. Statistical Analysis
5. Conclusions
Supplementary Information
ijms-15-05350-s001.pdfAcknowledgments
Conflicts of Interest
References
- Huang, S.; Frizzi, A.; Florida, C.A.; Kruger, D.E.; Luethy, M.H. High lysine and high tryptophan transgenic maize resulting from the reduction of both 19- and 22-kD alpha-zeins. Plant Mol. Biol 2006, 61, 525–535. [Google Scholar]
- Beauregard, M.; Hefford, M.A. Enhancement of essential amino acid contents in crops by genetic engineering and protein design. Plant Biotechnol. J 2006, 4, 561–574. [Google Scholar]
- Ferreira, R.R.; Varisi, V.A.; Meinhardt, L.W.; Lea, P.J.; Azevedo, R.A. Are high-lysine cereal crops still a challenge? Braz. J. Med. Biol. Res 2005, 38, 985–994. [Google Scholar]
- Tsai, C.; Dalby, A. Comparison of effect of shrunken-4, opaque-2, opaque-7, and floury-2 genes on zein content of maize during endosperm development. Cereal Chem 1974, 51, 825–829. [Google Scholar]
- Mertz, E.T.; Bates, L.S.; Nelson, O.E. Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science 1964, 145, 279–280. [Google Scholar]
- Sofi, P.A.; Wani, S.A.; Rather, A.G.; Wani, S.H. Review article: Quality protein maize (QPM): Genetic manipulation for the nutritional fortification of maize. J. Plant Breed. Crop. Sci 2009, 1, 244–253. [Google Scholar]
- Ortega, E.I.; Bates, L.S. Biochemical and agronomic studies of two modified hard-endosperm opaque-2 maize (Zea mays L.) populations. Cereal Chem 1983, 60, 107–111. [Google Scholar]
- Lambert, R.; Alexander, D.; Dudley, J. Relative performance of normal and modified protein (opaque-2) maize hybrids. Crop. Sci 1969, 9, 242–243. [Google Scholar]
- Quicke, G.V.; Gevers, H.O. Higher lysine levels and improved protein quality of opaque-2 maize. S. Afr. Med. J 1972, 46, 1579–1584. [Google Scholar]
- Larkins, B.A.; Lending, C.R.; Wallace, J.C. Modification of maize-seed-protein quality. Am. J. Clin. Nutr 1993, 58, 264S–269S. [Google Scholar]
- Scrimshaw, N.S. Quality protein maize. Food Nutr. Bull 2006, 27, 265–266. [Google Scholar]
- Vivek, B.S.; Krivanek, A.F.; Palacios-Rojas, N.; Twumasi-Afriyie, S.; Diallo, A.O. Breeding Quality Protein Maize (QPM): Protocols for Developing QPM Cultivars; CIMMYT: Mexico, D.F., Mexico, 2008. [Google Scholar]
- Scott, M.P.; Bhatnagar, S.; Betran, J. Tryptophan and methionine levels in quality protein maize breeding germplasm. Maydica 2004, 49, 303–311. [Google Scholar]
- Betran, F.J.; Bockholt, A.; Fojt, F.; Rooney, L.; Waniska, R. Registration of parental lines: Registration of Tx802. Crop. Sci 2003, 43, 1891–1892. [Google Scholar]
- Wang, M.; Liu, C.; Li, S.; Zhu, D.; Zhao, Q.; Yu, J. Improved nutritive quality and salt resistance in transgenic maize by simultaneously overexpression of a natural lysine-rich protein gene, SBgLR, and an ERF transcription factor gene, TSRF1. Int. J. Mol. Sci 2013, 14, 9459–9474. [Google Scholar]
- Segal, G.; Song, R.; Messing, J. A new opaque variant of maize by a single dominant RNA-interference-inducing transgene. Genetics 2003, 165, 387–397. [Google Scholar]
- Huang, S.; Adams, W.R.; Zhou, Q.; Malloy, K.P.; Voyles, D.A.; Anthony, J.; Kriz, A.L.; Luethy, M.H. Improving nutritional quality of maize proteins by expressing sense and antisense zein genes. J. Agric. Food Chem 2004, 52, 1958–1964. [Google Scholar]
- Frizzi, A.; Caldo, R.A.; Morrell, J.A.; Wang, M.; Lutfiyya, L.L.; Brown, W.E.; Malvar, T.M.; Huang, S. Compositional and transcriptional analyses of reduced zein kernels derived from the opaque2 mutation and RNAi suppression. Plant Mol. Biol 2010, 73, 569–585. [Google Scholar]
- Houmard, N.M.; Mainville, J.L.; Bonin, C.P.; Huang, S.; Luethy, M.H.; Malvar, T.M. High-lysine corn generated by endosperm-specific suppression of lysine catabolism using RNAi. Plant Biotechnol. J 2007, 5, 605–614. [Google Scholar]
- Frizzi, A.; Huang, S.; Gilbertson, L.A.; Armstrong, T.A.; Luethy, M.H.; Malvar, T.M. Modifying lysine biosynthesis and catabolism in corn with a single bifunctional expression/silencing transgene cassette. Plant Biotechnol. J 2008, 6, 13–21. [Google Scholar]
- Azevedo, R.A.; Arruda, P. High-lysine maize: The key discoveries that have made it possible. Amino Acids 2010, 39, 979–989. [Google Scholar]
- Reyes, A.R.; Bonin, C.P.; Houmard, N.M.; Huang, S.; Malvar, T.M. Genetic manipulation of lysine catabolism in maize kernels. Plant Mol. Biol 2009, 69, 81–89. [Google Scholar]
- Torrent, M.; Alvarez, I.; Geli, M.I.; Dalcol, I.; Ludevid, D. Lysine-rich modified gamma-zeins accumulate in protein bodies of transiently transformed maize endosperms. Plant Mol. Biol 1997, 34, 139–149. [Google Scholar]
- Sun, S.S.M.; Liu, Q.Q. Transgenic approaches to improve the nutritional quality of plant proteins. In Vitro Cell. Dev. Biol. Plant 2004, 40, 155–162. [Google Scholar]
- Ufaz, S.; Galili, G. Improving the content of essential amino acids in crop plants: Goals and opportunities. Plant Physiol 2008, 147, 954–961. [Google Scholar]
- Bicar, E.H.; Woodman-Clikeman, W.; Sangtong, V.; Peterson, J.M.; Yang, S.S.; Lee, M.; Scott, M.P. Transgenic maize endosperm containing a milk protein has improved amino acid balance. Transgenic Res 2008, 17, 59–71. [Google Scholar]
- Yu, J.; Peng, P.; Zhang, X.; Zhao, Q.; Zhu, D.; Sun, X.; Liu, J.; Ao, G. Seed-specific expression of the lysine-rich protein gene sb401 significantly increases both lysine and total protein content in maize seeds. Mol. Breed 2004, 14, 1–7. [Google Scholar]
- Lang, Z.; Zhao, Q.; Yu, J.; Zhu, D.; Ao, G. Cloning of potato SBgLR gene and its intron splicing in transgenic maize. Plant Sci 2004, 166, 1227–1233. [Google Scholar]
- Tang, M.; He, X.; Luo, Y.; Ma, L.; Tang, X.; Huang, K. Nutritional assessment of transgenic lysine-rich maize compared with conventional quality protein maize. J. Sci. Food Agric 2013, 93, 1049–1054. [Google Scholar]
- Ma, X. Activity Analysis of Millet (Setaria. italica) Seed-Specific Promoter pF128 in Maize (Zea. mays L.). Master Thesis, China Agricultural University, Department of Biochemistry and Molecular Biology, Beijing, China, May 2010. [Google Scholar]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: structures, properties and role in grain utilization. J. Exp. Bot 2002, 53, 947–958. [Google Scholar]
- Liu, C. Function and Application Study of the Potato Microtubule Associated Protein-SBgLR in Increasing Storage Protein Accumulation in Maize Seeds. Ph.D. Thesis, China Agricultural University, Department of Biochemistry and Molecular Biology, Beijing, China.
- Geetha, K.B.; Lending, C.R.; Lopes, M.A.; Wallace, J.C.; Larkins, B.A. opaque-2 modifiers increase gamma-zein synthesis and alter its spatial distribution in maize endosperm. Plant Cell 1991, 3, 1207–1219. [Google Scholar]
- Frame, B.R.; Shou, H.; Chikwamba, R.K.; Zhang, Z.; Xiang, C.; Fonger, T.M.; Pegg, S.E.; Li, B.; Nettleton, D.S.; Pei, D.; et al. Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol 2002, 129, 13–22. [Google Scholar]
- Beckwith, A.C.; Paulis, J.W.; Wall, J.S. Direct estimation of lysine in corn meals by the ninhydrin color reaction. J. Agric. Food Chem 1975, 23, 194–196. [Google Scholar]
- Osborne, B.G. Principle and practice of near infra-red (NIR) reflectance analysis. Int. J. Food Sci. Technol 1981, 16, 13–19. [Google Scholar]
- Wallace, J.C.; Lopes, M.A.; Paiva, E.; Larkins, B.A. New methods for extraction and quantitation of zeins reveal a high content of γ-Zein in modified opaque-2 Maize. Plant Physiol 1990, 92, 191–196. [Google Scholar]




| Line | Lysine content (g/100 g seed) | Protein content (g/100 g seed) |
|---|---|---|
| F12 | 0.37 ± 0.01* | 12.36 ± 0.5* |
| F13 | 0.38 ± 0.01* | 10.20 ± 0.3 ns |
| F17 | 0.35 ± 0.01* | 9.93 ± 0.4 ns |
| F19 | 0.46 ± 0.02** | 10.40 ± 0.3 ns |
| F28 | 0.39 ± 0.02* | 10.38 ± 0.6 ns |
| F33 | 0.37 ± 0.03* | 10.23 ± 0.2 ns |
| F34 | 0.35 ± 0.03* | 9.54 ± 0.3 ns |
| F35 | 0.36 ± 0.01* | 10.01 ± 0.2 ns |
| F38 | 0.40 ± 0.03* | 9.89 ± 0.5 ns |
| F45 | 0.35 ± 0.03* | 11.65 ± 0.7* |
| F47 | 0.37 ± 0.01* | 11.79 ± 0.6* |
| F50 | 0.36 ± 0.02* | 10.09 ± 0.2 ns |
| F52 | 0.35 ± 0.01* | 9.79 ± 0.3 ns |
| F53 | 0.37 ± 0.02* | 10.26 ± 0.4 ns |
| F57 | 0.36 ± 0.01* | 10.47 ± 0.6 ns |
| F64 | 0.35 ± 0.01* | 10.07 ± 0.2 ns |
| F72 | 0.37 ± 0.01* | 10.18 ± 0.1 ns |
| F73 | 0.43 ± 0.02** | 10.58 ± 0.6 ns |
| F77 | 0.38 ± 0.02* | 10.32 ± 0.4 ns |
| F78 | 0.39 ± 0.01* | 11.09 ± 0.6* |
| F83 | 0.37 ± 0.01* | 10.50 ± 0.4 ns |
| F84 | 0.38 ± 0.02* | 10.77 ± 0.6 ns |
| F87 | 0.36 ± 0.01* | 11.47 ± 0.8* |
| F88 | 0.40 ± 0.01* | 11.56 ± 0.7* |
| F89 | 0.37 ± 0.02* | 9.75 ± 0.7 ns |
| F91 | 0.37 ± 0.01* | 10.02 ± 0.4 ns |
| WTa | 0.28 ± 0.01 | 10.19 ± 0.3 |
| Line | Lysine content (g/100 g seed) | Protein content (g/100 g seed) | ||||
|---|---|---|---|---|---|---|
| T2 | T3 | T4 | T2 | T3 | T4 | |
| F12 | 0.39 ± 0.02* | 0.37 ± 0.01* | 0.38 ± 0.01* | 13.17 ± 0.5* | 12.40 ± 0.1* | 12.55 ± 0.2* |
| F78 | 0.38 ± 0.01* | 0.39 ± 0.01* | 0.40 ± 0.01* | 12.31 ± 0.2* | 12.29 ± 0.5* | 13.15 ± 0.5** |
| F88 | 0.41 ± 0.01* | 0.41 ± 0.02* | 0.44 ± 0.02** | 12.46 ± 0.4* | 12.40 ± 0.2* | 12.70 ± 0.1* |
| WTa | 0.26 ± 0.02 | 0.30 ± 0.01 | 0.31 ± 0.01 | 9.93 ± 0.1 | 10.11 ± 0.2 | 10.20 ± 0.2 |
| Line | Lipid content (g/100 g seed) | Starch content (g/100 g seed) | ||
|---|---|---|---|---|
| T2 | T3 | T2 | T3 | |
| F12 | 4.35 ± 0.31 | 4.04 ± 0.10 | 65.34 ± 0.95 | 69.61 ± 0.17 |
| F78 | 4.68 ± 0.22 | 4.46 ± 0.25 | 65.82 ± 1.10 | 68.96 ± 1.69 |
| F88 | 4.05 ± 0.14 | 4.32 ± 0.14 | 66.14 ± 0.54 | 71.69 ± 1.20 |
| WTa | 4.50 ± 0.50 | 4.03 ± 0.12 | 69.10 ± 0.32 | 72.25 ± 0.84 |
| Line | PH (cm) | EH (cm) | EL (cm) | BTL (cm) | ED (cm) | ER | KW (g) |
|---|---|---|---|---|---|---|---|
| F12 | 213.6 ± 6.7 | 100.3 ± 4.5 | 9.5 ± 2.4 | 2.4 ± 1.4 | 40.6 ± 3.6 | 14 | 29.3 ± 6.1 |
| F78 | 195.8 ± 5.5 | 98.2 ± 5.2 | 10.2 ± 2.3 | 0.5 ± 0.4 | 38.7 ± 3.9 | 16 | 26.8 ± 7.4 |
| F88 | 200.5 ± 7.8 | 90.3 ± 3.2 | 7.9 ± 1.4 | 1.4 ± 1.2 | 37.2 ± 1.5 | 16 | 20.4 ± 0.3 |
| WTa | 203.5 ± 8.3 | 87.6 ± 5.4 | 11.3 ± 1.5 | 0.9 ± 0.6 | 42.3 ± 2.6 | 14 | 27.3 ± 2.0 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yue, J.; Li, C.; Zhao, Q.; Zhu, D.; Yu, J. Seed-Specific Expression of a Lysine-Rich Protein Gene, GhLRP, from Cotton Significantly Increases the Lysine Content in Maize Seeds. Int. J. Mol. Sci. 2014, 15, 5350-5365. https://doi.org/10.3390/ijms15045350
Yue J, Li C, Zhao Q, Zhu D, Yu J. Seed-Specific Expression of a Lysine-Rich Protein Gene, GhLRP, from Cotton Significantly Increases the Lysine Content in Maize Seeds. International Journal of Molecular Sciences. 2014; 15(4):5350-5365. https://doi.org/10.3390/ijms15045350
Chicago/Turabian StyleYue, Jing, Cong Li, Qian Zhao, Dengyun Zhu, and Jingjuan Yu. 2014. "Seed-Specific Expression of a Lysine-Rich Protein Gene, GhLRP, from Cotton Significantly Increases the Lysine Content in Maize Seeds" International Journal of Molecular Sciences 15, no. 4: 5350-5365. https://doi.org/10.3390/ijms15045350




