Epigallocatechin-3-O-(3-O-methyl)-gallate-induced Differentiation of Human Keratinocytes Involves Klotho-Mediated Regulation of Protein Kinase-cAMP Responsive Element-Binding Protein Signaling
Abstract
:1. Introduction
2. Results
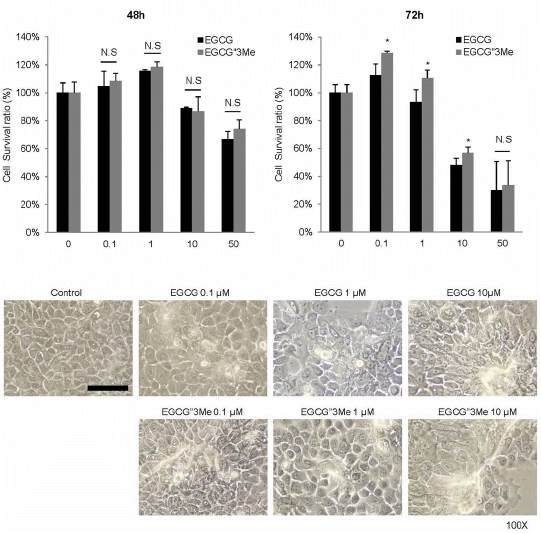
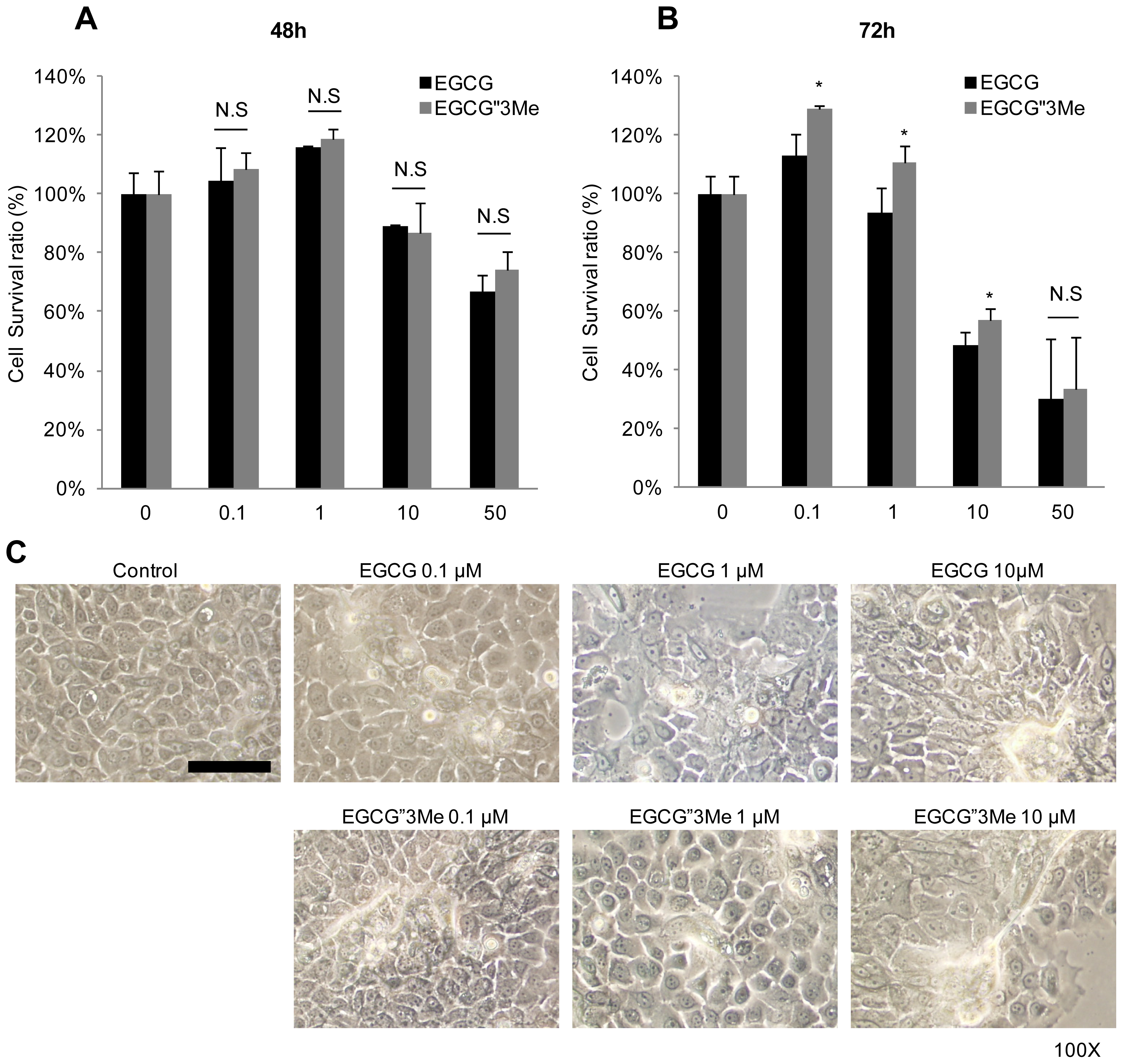
2.1. EGCG″3Me Induces Keratinocyte Differentiation
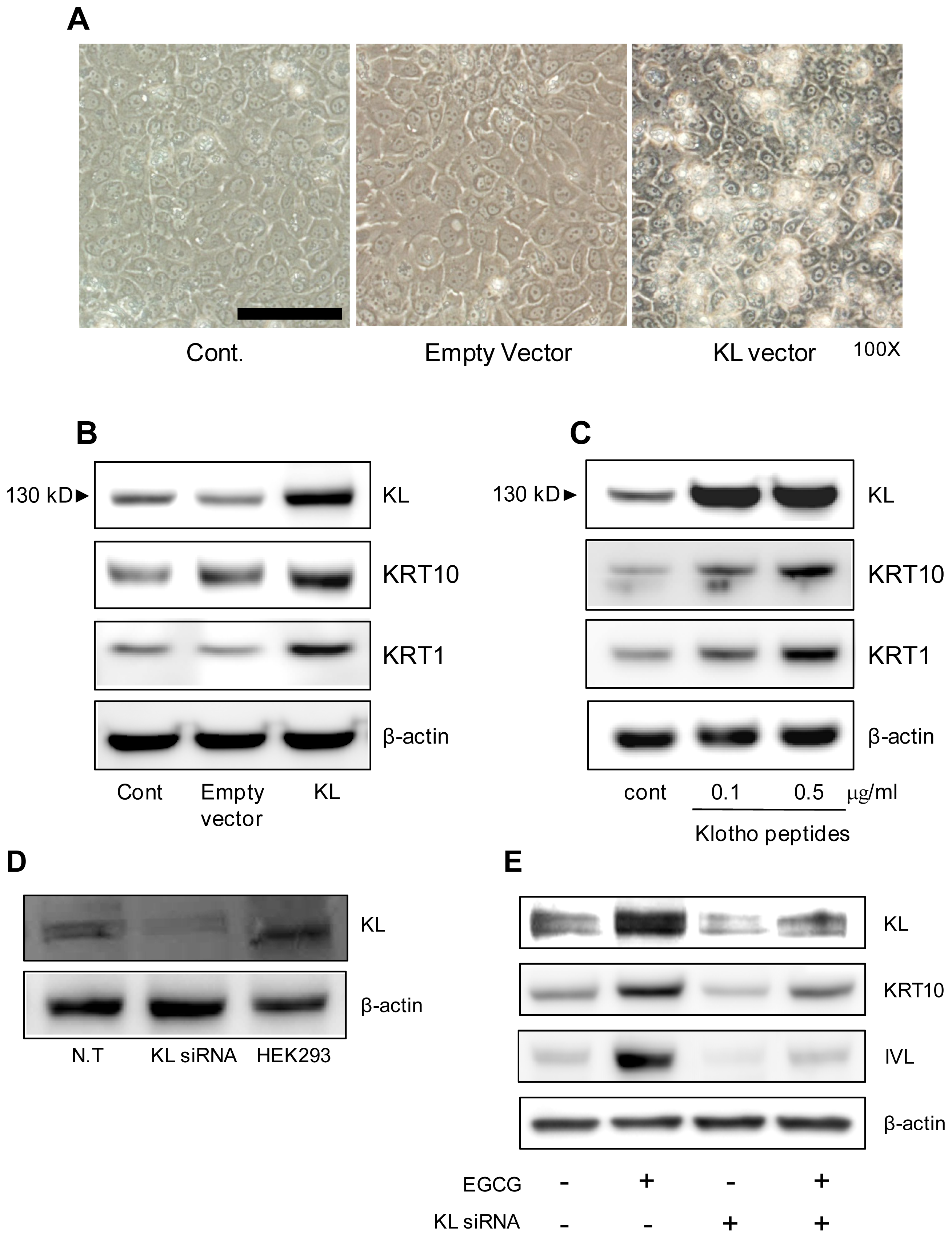
2.2. EGCG″3Me Increases KL Expression
2.3. KL Increases Keratinocyte Differentiation Markers
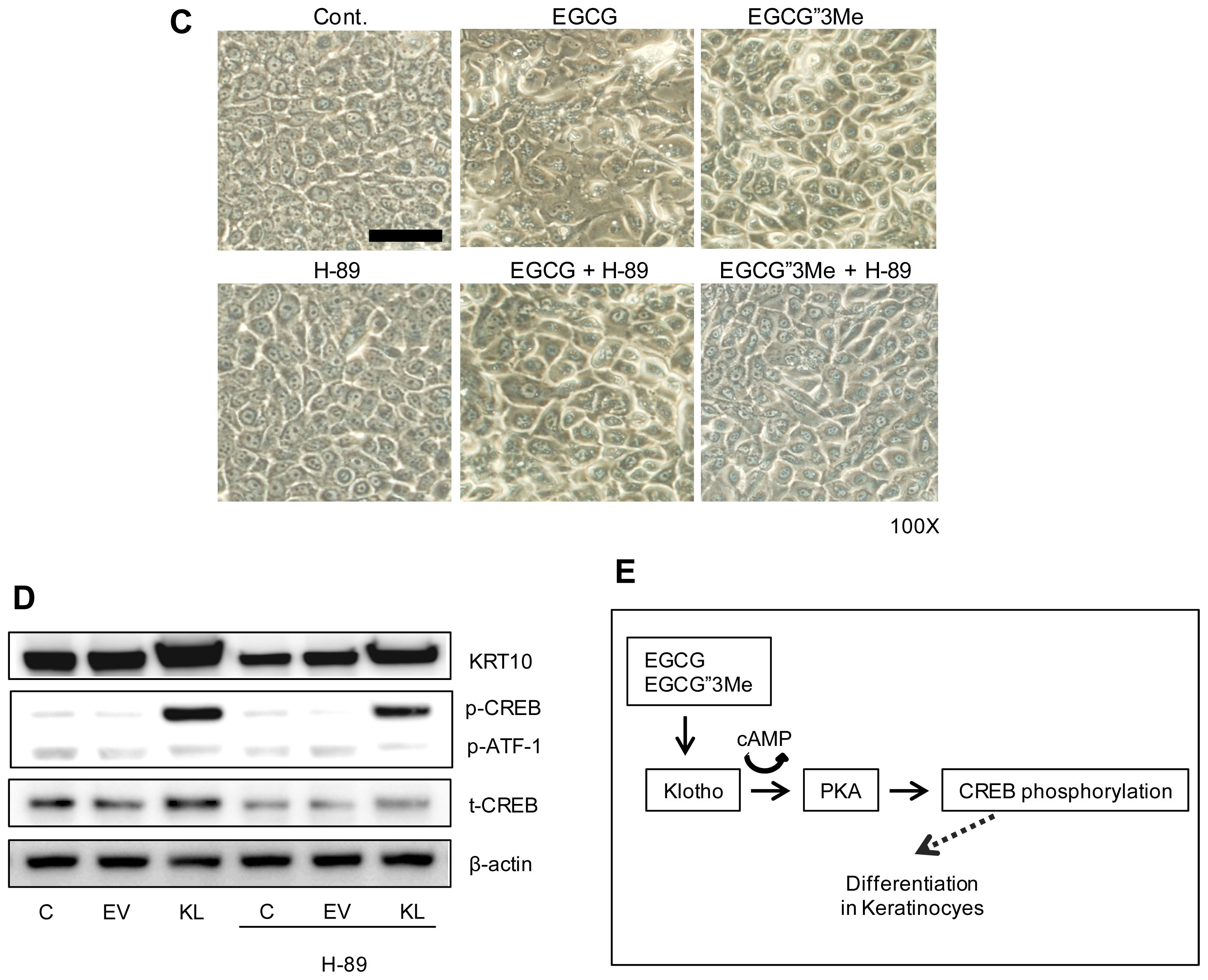
2.4. Morphological Changes in NHEKs Induced by EGCG and EGCG″3Me Involve PKA Signaling and Are Inhibited by the PKA Inhibitor H-89
3. Discussion
4. Experimental Procedures
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. RNA Extraction and Quantitative Real-Time RT-PCR (qRT-PCR)
4.5. Knock-Down or Over-Expression of KL
4.6. Western Blot Analysis
4.7. Statistical Analyses
5. Conclusions
Conflicts of Interest
- Author ContributionsConceived and designed the experiments: H.-J.K., H.C., S.H.H., D.W.S. and H.K. Performed the experiments: H.-J.K., H.C., S.H.H., J.-Y.J. and S.A. Analyzed the data: H.-J.K., H.C., D.W.S., H.K., M.S.L., S.-Y.B., J.H.L., J.H.L. and T.R.L. Contributed reagents/materials/analysis tools: S.A., M.S.L., S.-Y.B., J.H.L. and J.H.L. Wrote the paper: H.-J.K., H.C., S.H.H., T.R.L., D.W.S. and H.K.
References
- Holbrook, K. Ultrastructure of the epidermis. In The Keratinocyte Handbook, 1st ed.; Leigh, I., Lane, B., Watt, F., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 33–39. [Google Scholar]
- Haake, A.R.; Holbrook, K.; Freedberg, I.M.; Eisen, A.Z.; Wolff, K.; Austen, K.F.; Goldsmith, L.A.; Katz, S.I.; Fitzpatrik, T.B. The structure and development of skin. In Fitzpatrick’s Dermatology in General Medicine; McGraw-Hill: New York, NY, USA, 1999; pp. 70–114. [Google Scholar]
- Nakatani, T.; Sarraj, B.; Ohnishi, M.; Densmore, M.J.; Taguchi, T.; Goetz, R.; Mohammadi, M.; Lanske, B.; Razzaque, M.S. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (FGF23)-mediated regulation of systemic phosphate homeostasis. FASEB J 2009, 23, 433–441. [Google Scholar]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci 2007, 81, 519–533. [Google Scholar]
- Yang, C.S.; Maliakal, P.; Meng, X. Inhibition of carcinogenesis by tea. Annu. Rev. Pharmacol. Toxicol 2002, 42, 25–54. [Google Scholar]
- Mukhtar, H.; Ahmad, N. Tea polyphenols: Prevention of cancer and optimizing health. Am. J. Clin. Nutr 2000, 71 Suppl 6, 1698S–1702S. [Google Scholar]
- Wang, Z.Y.; Cheng, S.J.; Zhou, Z.C.; Athar, M.; Khan, W.A.; Bickers, D.R.; Mukhtar, H. Antimutagenic activity of green tea polyphenols. Mutat. Res 1989, 223, 273–285. [Google Scholar]
- Cao, Y.; Cao, R. Angiogenesis inhibited by drinking tea. Nature 1999, 398, 381. [Google Scholar]
- Yang, T.T.; Koo, M.W. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion. Life Sci 2000, 66, 411–423. [Google Scholar]
- Shutsung, L.; Yung, K.; Hiipakka, R.A. Green tea: Biochemical and biological basis for health benefits. Vitam. Horm 2001, 62, 1–94. [Google Scholar]
- Chung, J.H.; Han, J.H.; Hwang, E.J.; Seo, J.Y.; Cho, K.H.; Kim, K.H.; Youn, J.I.; Eun, H.C. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J 2003, 17, 1913–1915. [Google Scholar]
- Gianeti, M.D.; Mercurio, D.G.; Campos, P.M. The use of green tea extract in cosmetic formulations: Not only an antioxidant active ingredient. Dermatol. Ther 2013, 26, 267–271. [Google Scholar]
- Scalia, S.; Marchetti, N.; Bianchi, A. Comparative evaluation of different co-antioxidants on the photochemical- and functional-stability of epigallocatechin-3-gallate in topical creams exposed to simulated sunlight. Molecules 2013, 18, 574–587. [Google Scholar]
- Feng, W.Y. Metabolism of green tea catechins: An overview. Curr. Drug Metab 2006, 7, 755–809. [Google Scholar]
- Sang, S.; Lambert, J.D.; Ho, C.T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res 2011, 64, 87–99. [Google Scholar]
- Masazumi, S.; Kyoji, Y.; Mari, M.Y.; Toshio, M.; Mitsuaki, S. Inhibitory effects of tea catechins and O-methylated derivatives of (−)-epigallocatechin-3-O-gallate on mouse type IV allergy. J. Agric. Food Chem 2000, 48, 5649–5653. [Google Scholar]
- Kyoji, Y.; Kenjiro, O.; Toshio, M.; Mitsuaki, S. Inhibitory effects of the C-2 epimeric isomers of tea catechins on mouse type IV allergy. J. Agric. Food Chem 2004, 52, 4660–4663. [Google Scholar]
- Hsu, S. Green tea and the skin. J. Am. Acad. Dermatol 2005, 52, 1049–1059. [Google Scholar]
- Hsu, S.; Bollag, W.B.; Lewis, J.; Huang, Q.; Singh, B.; Sharawy, M.; Yamamoto, T.; Schuster, G. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. J. Pharmacol. Exp. Ther 2003, 306, 29–34. [Google Scholar]
- Liu, L.; Xie, H.; Chen, X.; Shi, W.; Xiao, X.; Lei, D.; Li, J. Differential response of normal human epidermal keratinocytes and HaCaT cells to hydrogen peroxide-induced oxidative stress. Clin. Exp. Dermatol 2012, 37, 772–780. [Google Scholar]
- Hiroshi, K.; Masaya, Y.; Makoto, K. Suppression of aging in mice by the hormone klotho. Science 2005, 309, 1829–1833. [Google Scholar]
- Meinkoth, J.L.; Alberts, A.S.; Went, W.; Fantozzi, D.; Taylor, S.S.; Hagiwara, M.; Montminy, M.; Feramisco, J.R. Signal transduction through the cAMP-dependent protein kinase. Mol. Cell. Biochem 1993, 127–128, 179–186. [Google Scholar]
- Shaywitz, A.J.; Michael, E.G. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem 1999, 68, 821–861. [Google Scholar]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol 2001, 2, 599–609. [Google Scholar]
- Mammone, T.; Marenus, K.; Maes, D.; Lockshin, R.A. The induction of terminal differentiation markers by the cAMP pathway in human HaCaT keratinocytes. Skin Pharmacol. Appl. Skin Physiol 1998, 11, 152–160. [Google Scholar]
- Yang, J.; Matsukawa, N.; Rakugi, H.; Imai, M.; Kida, I.; Nagai, M.; Ohta, J.; Fukuo, K.; Nabeshima, Y.; Ogihara, T. Upregulation of cAMP is a new functional signal pathway of Klotho in endothelial cells. Biochem. Biophys. Res. Commun 2003, 301, 424–429. [Google Scholar]
- Kokkinaki, M.; Abu-Asab, M.; Gunawardena, N.; Ahern, G.; Javidnia, M.; Young, J.; Golestaneh, N. Klotho regulates retinal pigment epithelial functions and protects against oxidative stress. J. Neurosci 2013, 33, 16346–16359. [Google Scholar]
- Li, L.; Tucker, R.W.; Hennings, H.; Yuspa, S.H. Inhibitors of the intracellular Ca(2+)-ATPase in cultured mouse keratinocytes reveal components of terminal differentiation that are regulated by distinct intracellular Ca2+ compartments. Cell Growth Differ 1995, 6, 1171–1184. [Google Scholar]
- Li, L.; Tucker, R.W.; Hennings, H.; Yuspa, S.H. Chelation of intracellular Cax2+ inhibits murine keratinocyte differentiation in vitro. J. Cell. Physiol. 1995, 163, 105–114. [Google Scholar]
- Hennings, H.; Kruszewski, F.H.; Yuspa, S.H.; Tucker, R.W. Intracellular calcium alterations in response to increased external calcium in normal and neoplastic keratinocytes. Carcinogenesis 1989, 10, 777–780. [Google Scholar]
- Kruszewski, F.H.; Hennings, H.; Yuspa, S.H.; Tucker, R.W. Regulation of intracellular free calcium in normal murine keratinocytes. Am. J. Physiol 1991, 261, C767–C773. [Google Scholar]
- Balasubramanian, S.; Sturniolo, M.T.; Dubyak, G.R.; Eckert, R.L. Human epidermal keratinocytes undergo (−)-epigallocatechin-3-gallate-dependent differentiation but not apoptosis. Carcinogenesis 2005, 26, 1100–1108. [Google Scholar]



© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, H.-J.; Chang, H.; Han, S.H.; Lee, M.S.; Jung, J.-Y.; An, S.; Baek, S.-Y.; Lee, J.H.; Lee, J.H.; Lee, T.R.; et al. Epigallocatechin-3-O-(3-O-methyl)-gallate-induced Differentiation of Human Keratinocytes Involves Klotho-Mediated Regulation of Protein Kinase-cAMP Responsive Element-Binding Protein Signaling. Int. J. Mol. Sci. 2014, 15, 5749-5761. https://doi.org/10.3390/ijms15045749
Kim H-J, Chang H, Han SH, Lee MS, Jung J-Y, An S, Baek S-Y, Lee JH, Lee JH, Lee TR, et al. Epigallocatechin-3-O-(3-O-methyl)-gallate-induced Differentiation of Human Keratinocytes Involves Klotho-Mediated Regulation of Protein Kinase-cAMP Responsive Element-Binding Protein Signaling. International Journal of Molecular Sciences. 2014; 15(4):5749-5761. https://doi.org/10.3390/ijms15045749
Chicago/Turabian StyleKim, Hyoung-June, Huikyoung Chang, Seung Hun Han, Min Seuk Lee, Ji-Yong Jung, SoonAe An, Seok-Yun Baek, Jin Ho Lee, John Hwan Lee, Tae Ryong Lee, and et al. 2014. "Epigallocatechin-3-O-(3-O-methyl)-gallate-induced Differentiation of Human Keratinocytes Involves Klotho-Mediated Regulation of Protein Kinase-cAMP Responsive Element-Binding Protein Signaling" International Journal of Molecular Sciences 15, no. 4: 5749-5761. https://doi.org/10.3390/ijms15045749