Molecular Imprinted Polymer of Methacrylic Acid Functionalised ?-Cyclodextrin for Selective Removal of 2,4-Dichlorophenol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of MIP MAA-βCD and NIP MAA-βCD
2.1.1. Fourier Transform Infrared (FTIR) Analysis
2.1.2. Particle Size and Brunauer-Emmett-Teller (BET) Analysis
2.1.3. Morphological Structure
2.2. Effect of pH on the Adsorption of 2,4-DCP
2.3. Effect of Initial 2,4-DCP Concentrations on the Adsorption Process
2.4. Adsorption Isotherms
2.5. Adsorption Kinetics
2.5.1. Adsorption Kinetic Models
2.5.2. Validation of Kinetic Models
2.5.3. Intraparticle Diffusion
2.5.4. External Diffusion
2.6. Adsorption Thermodynamics
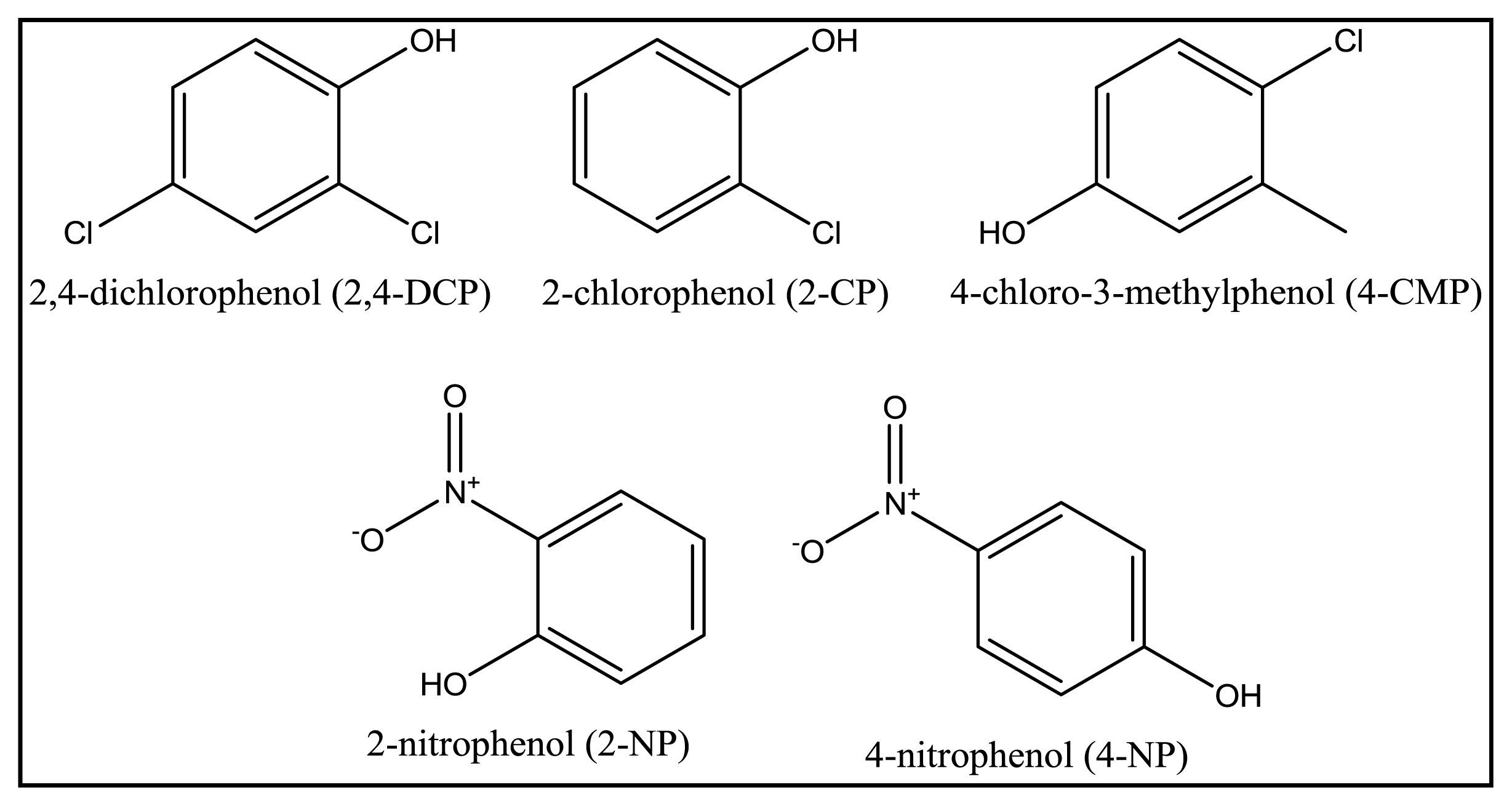
2.7. Selective Adsorption of MIP MAA-βCD towards 2,4-DCP
2.8. Comparison with Other Adsorbents
2.9. Possible Interaction between 2,4-DCP and MAA-βCD
3. Experimental Section
3.1. Chemicals
3.2. Instruments
3.3. Synthesis of MAA-βCD Monomer
3.4. Synthesis of MIP MAA-βCD and NIP MAA-βCD
3.5. Batch Adsorption Studies
3.6. Selectivity Experiments
4. Conclusions
Supplementary Information
ijms-15-06111-s001.pdfAcknowledgments
Conflicts of Interest
References
- Sahiner, N.; Ozay, O.; Aktas, N. Aromatic organic contaminant removal from an aqueous environment by p (4-VP)-based materials. Chemosphere 2011, 85, 832–838. [Google Scholar]
- Feng, Q.Z.; Zhao, L.X.; Yan, W.; Lin, J.M.; Zheng, Z.X. Molecularly imprinted solid-phase extraction combined with high performance liquid chromatography for analysis of phenolic compounds from environmental water samples. J. Hazard. Mater 2009, 167, 282–288. [Google Scholar]
- Antizar-Ladislao, B.; Galil, N.I. Biosorption of phenol and chlorophenols by acclimated residential biomass under bioremediation conditions in a sandy aquifer. Water Res 2004, 38, 267–276. [Google Scholar]
- Radhika, M.; Palanivelu, K. Adsorptive removal of chlorophenols from aqueous solution by low cost adsorbent-Kinetics and isotherm analysis. J. Hazard. Mater. B 2006, 138, 116–124. [Google Scholar]
- Keith, L.H.; Telliard, W.A. Priority pollutants: I-a perspective view. Environ. Sci. Technol 1979, 13, 416–423. [Google Scholar]
- Lin, C.H.; Tseng, S.K. Electrochemically reductive dechlorination of pentachlorophenol using a high overpotential zinc cathode. Chemosphere 1999, 39, 2375–2389. [Google Scholar]
- Ersoz, A.; Denizli, A.; Sener, I.; Atilir, A.; Diltemiz, S.; Say, R. Removal of phenolic compounds with nitrophenol-imprinted polymer based on π-π and hydrogen-bonding interactions. Sep. Purif. Technol 2004, 38, 173–179. [Google Scholar]
- Bukowska, B. Effects of 2, 4-D and its metabolite 2, 4-dichlorophenol on antioxidant enzymes and level of glutathione in human erythrocytes. Comp. Biochem. Physiol 2003, 135, 435–441. [Google Scholar]
- Wang, J.P.; Chen, Y.Z.; Feng, H.M.; Zhang, S.J.; Yu, H.Q. Removal of 2,4-dichlorophenol from aqueous solution by static-air-activated carbon fibers. J. Colloid Interface Sci 2007, 313, 80–85. [Google Scholar]
- Denizli, A.; Ozkan, G.; Ucar, M. Removal of chlorophenols from aquatic systems with dye-affinity microbeads. Sep. Purif. Technol 2001, 24, 255–262. [Google Scholar]
- Mahajan, S.P. Pollution Control in Process Industries; Tata McGraw-Hill: New Delhi, India, 1991; p. 18. [Google Scholar]
- Rengaraj, S.; Moon, S.H.; Sivabalan, R.; Arabindoo, B.; Murugesan, V. Agricultural solid waste for the removal of organics adsorption of phenol from water and wastewater by palm seed coat activated carbon. Waste Manag 2002, 22, 543–548. [Google Scholar]
- Klumpp, E.; Contreras-Ortega, C.; Klahre, P.; Tino, F.J.; Yapar, S.; Portillo, C.; Stegen, S.; Queirolo, F.; Schwuger, M.J. Sorption of 2,4-dichlorophenol on modified hydrotalcites. Colloids Surf. A 2003, 230, 111–116. [Google Scholar]
- Shu, H.T.; Li, D.; Scala, A.A.; Ma, Y.H. Adsorption of small organic pollutants from aqueous streams by aluminosilicate-based microporous materials. Sep. Purif. Technol 1997, 11, 27–36. [Google Scholar]
- Gallizia, I.; McClean, S.; Banat, I.B. Bacterial biodegradation of phenol and 2,4-dichlorophenol. J. Chem. Tech. Biotech 2003, 78, 959–963. [Google Scholar]
- Wang, C.C.; Lee, C.M.; Kuan, C.H. Removal of 2,4-dichlorophenol by suspended and immobilized. Bacillus insolitus. Chemosphere 2000, 41, 447–452. [Google Scholar]
- Pera-Titus, M.; Garcia-Molina, V.; Banos, M.A.; Gimenez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: a general review. Appl. Catal. B 2004, 47, 219–256. [Google Scholar]
- Koyama, O.; Kamagat, Y.; Nakamura, K. Degradation of chlorinated aromatics by Fenton oxidation and methanogenic digester sludge. Water Res 1994, 28, 895–899. [Google Scholar]
- Fukushima, M.; Tatsumi, K. Degradation pathways of pentachlorophenol by photo-Fenton systems in the presence of iron(III), humic acid and hydrogen peroxide. Environ. Sci. Technol 2001, 35, 1771–1778. [Google Scholar]
- Esplugas, S.; Gimenez, J.; Contreras, S.; Pascual, E.; Rodriguez, M. Comparison of different advanced oxidation processes for phenol degradation. Water Res 2002, 36, 1034–1042. [Google Scholar]
- Dutta, S.; Basu, J.K.; Ghar, R.N. Studies on adsorption of p-nitrophenol on charred saw-dust. Sep. Purif. Technol 2001, 21, 227–235. [Google Scholar]
- Chin, Y.K.; Mohamad, S.; Abas, M.R. Removal of parabens from aqueous solution using β-cyclodextrin cross-linked polymer. Int. J. Mol. Sci 2010, 11, 3459–3471. [Google Scholar]
- Furuya, E.G.; Chang, H.T.; Miura, Y.; Noll, K.E. A fundamental analysis of the isotherm for the adsorption of phenolic compounds on activated carbon. Sep. Purif. Technol 1997, 11, 69. [Google Scholar]
- Wu, J.; Yu, H.Q. Biosorption of 2,4-dichlorophenol from aqueous solution by Phanerochaete chrysosporium biomass: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. B 2006, 137, 498–508. [Google Scholar]
- Li, Y.; Li, X.; Li, Y.; Qi, J.; Bian, J.; Yuan, Y. Selective removal of 2,4-dichlorophenol from contaminated water using non-covalent imprinted microspheres. Environ. Pollut 2009, 157, 1879–1885. [Google Scholar]
- Yan, H.; Row, K.H. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci 2006, 7, 155–178. [Google Scholar]
- Suedee, R.; Seechamnanturakit, V.; Suksuwan, A.; Canyuk, B. Recognition Pproperties and competitive assays of a dual dopamine/serotonin selective molecularly imprinted polymer. Int. J. Mol. Sci 2008, 9, 2333–2356. [Google Scholar]
- Valero-Navarro, A.; Salinas-Castillo, A.; Fernandez-Sanchez, J.F.; Segura-Carretero, A.; Mallavia, R.; Fernandez-Gutierrez, A. The development of a MIP MAA-β-CD-optosensor for the detection of monoamine naphthalenes in drinking water. Biosens. Bioelectron 2009, 24, 2305–2311. [Google Scholar]
- Asman, S.; Yusof, N.A.; Abdullah, A.H.; Haron, M.J. Synthesis and characterization of a molecularly imprinted polymer for methylene blue. Asian J. Chem 2011, 23, 4786–4794. [Google Scholar]
- Abdul Raof, S.F.; Mohamad, S.; Abas, M.R. Synthesis and evaluation of molecularly imprinted silica gel for 2-hydroxybenzoic acid in aqueous solution. Int. J. Mol. Sci 2013, 14, 5952–5965. [Google Scholar]
- Guo, L.; Deng, Q.; Fang, G.; Gao, W.; Shuo, W. Preparation and evaluation of molecularly imprinted ionic liquids polymer as sorbent for on-line solid-phase extraction of chlorsulfuron in environmental water samples. J. Chromatogr. A 2011, 1218, 6271–6277. [Google Scholar]
- Mohamad, S.; Surikumaran, H.; Raoov, M.; Marimuthu, T.; Chandrasekaram, K.; Subramaniam, P. Conventional study on novel dicationic ionic liquid inclusion with β-cyclodextrin. Int. J. Mol. Sci 2011, 12, 6329–6345. [Google Scholar]
- Suzuki, I.; Egawa, Y.; Mizukawa, Y.; Hoshi, T.; Anzai, J. Construction of positively-charged layered assemblies assisted by cyclodextrin complexation. Chem. Commun 2002, 13, 164–165. [Google Scholar]
- Pariot, N.; Edwards-Levy, F.; Andry, M.C.; Levy, M.C. Cross-linked β-cyclodextrin microcapsules. II. Retarding effect on drug release through semi-permeable membranes. Int. J. Pharm 2002, 232, 175–181. [Google Scholar]
- Garcia-Zubiri, I.X.; Gonzalez-Gaitano, G.; Isasi, J.R. Isosteric heats of sorption of 1-naphthol and phenol from aqueous solutions by β-cyclodextrin polymers. J. Colloid Interface Sci 2007, 307, 64–70. [Google Scholar]
- Pan, J.; Zou, X.; Wang, X.; Guan, W.; Yan, Y.; Han, J. Selective recognition of 2,4-dichlorophenol from aqueous solution by uniformly sized molecularly imprinted microspheres with β-cyclodextrin/attapulgite composites as support. Chem. Eng. J 2010, 162, 910–918. [Google Scholar]
- Li, Y.; Li, X.; Dong, C.; Li, Y.; Jin, P.; Qi, J. Selective recognition and removal of chlorophenols from aqueous solution using molecularly imprinted polymer prepared by reversible addition-fragmentation chain transfer polymerization. Biosens. Bioelectron 2009, 25, 306–312. [Google Scholar]
- Wang, L.; Zhang, J.; Zhao, R.; Zhang, C.; Li, C.; Li, Y. Adsorption of 2,4-dichlorophenol on Mn-modified activated carbon prepared from Polygonum orientale Linn. Desalination 2011, 266, 175–181. [Google Scholar]
- Li, N.; Mei, Z.; Ding, S.G. 2,4-Dichlorophenol sorption on cyclodextrin polymers. J. Incl. Phenom. Macrocycl. Chem 2010, 68, 123–129. [Google Scholar]
- Leyva, E.; Moctezuma, E.; Strouse, J.; Garcia-Garibay, M.A. Spectrometric and 2D NMR studies on the complexation of chlorophenols with cyclodextrins. J. Incl. Phenom. Macrocycl. Chem 2001, 39, 41–46. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc 1916, 38, 2221–2295. [Google Scholar]
- Freundlich, H.M.F. U ber die adsorption in losungen. Z. Phys.Chem 1906, 57, 385–470. [Google Scholar]
- Temkin, M.J.; Phyzev, V. Recent modifications to Langmuir isotherms. Acta Phys. Chim 1940, 12, 217–222. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Chem. Zentr 1947, 1, 875. [Google Scholar]
- Halif, N.A.; Daud, W.M.A.W.; Noor, I.M.; Hassan, C.R.C. The effect of temperature on the adsorption of 4-nitrophenol onto palm shell based activated carbon. AEESEAP J 2007, 31, 23–29. [Google Scholar]
- Weber, T.W.; Chakkravorti, R.K. Pore and solid diffusion models for fixed bed absorbers. Am. Inst. Chem. Eng. J 1974, 20, 228. [Google Scholar]
- Akhtar, M.; Bhanger, M.I.; Iqbal, S.; Hasany, S.M. Sorption potential of rice husk for the removal of 2,4-dichlorophenol from aqueous solutions: Kinetic and thermodynamic investigations. J. Hazard. Mater. B 2006, 128, 44–52. [Google Scholar]
- Langergen, S.; Svenska, B.K. About the theory of so-called adsorption of soluble substances. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem 1999, 34, 451–465. [Google Scholar]
- Castro Lopez, M.M.; Cela Perez, M.C.; Dopico Garcia, M.S.; Lopez Vilarino, J.S.; Gonzalez Rodriguez, M.V.; Barral Losada, L.F. Preparation, evaluation and characterization of quercetin-molecularly imprinted polymer for preconcentration and clean-up of catechins. Anal. Chim. Acta 2012, 721, 68–78. [Google Scholar]
- Shaarani, F.W.; Hameed, B.H. Ammonia-modified activated carbon for the adsorption of 2,4-dichlorophenol. Chem. Eng. J 2011, 169, 180–185. [Google Scholar]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci 2008, 143, 48–67. [Google Scholar]
- Bhatnagar, A.; Minocha, A.K. Adsorptive removal of 2,4-dichlorophenol from water utilizing Punica granatum peel waste and stabilization with cement. J. Hazard. Mater 2009, 168, 1111–1117. [Google Scholar]
- Ahmed, M.J.; Theydan, S.K. Equilibrium isotherms, kinetics and thermodynamics studies of phenolic compounds adsorption on palm-tree fruit stones. Ecotoxic. Environ. Saf 2012, 84, 39–45. [Google Scholar]
- Aroua, M.K.; Leong, S.P.P.; Teo, L.Y.; Daud, W.M.A.W. Real time determination of kinetics of adsorption of lead(II) onto shell based activated carbon using ion selection electrode. Bioresour. Technol 2008, 99, 5786–5792. [Google Scholar]
- Liu, Q.S.; Zheng, T.; Wang, P.; Jiang, J.P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J 2010, 157, 348–356. [Google Scholar]
- Rojas, G.; Silva, J.; Flores, J.; Rodriguez, A.; Ly, M.; Maldonado, H. Adsorption of chromium onto cross-linked chitosan. Sep. Purif. Technol 2005, 44, 31–36. [Google Scholar]
- Chang, Y.; Ko, T.; Hsu, T.; Syu, M. Synthesis of an imprinted hybrid organic-inorganic polymeric sol-gel matrix toward the specific binding and isotherm kinetics investigation of creatinine. Anal. Chem 2009, 81, 2098–2105. [Google Scholar]
- Sathishkumar, M.; Binupriya, A.R.; Kavitha, D.; Selvakumar, R.; Jayabalan, R.; Choi, J.G.; Yun, S.E. Adsorption potential of maize cob carbon for 2,4-dichlorophenol removal from aqueous solutions: equilibrium, kinetics and thermodynamics modeling. Chem. Eng. J 2009, 147, 265–271. [Google Scholar]
- Sathishkumar, M.; Binupriya, A.R.; Kavitha, D.; Yun, S.E. Kinetic and isothermal studies on liquid-phase adsorption of 2,4-dichlorophenol by palm pith carbon. Bioresour. Technol 2007, 98, 866–873. [Google Scholar]
- Alam, M.Z.; Muyibi, S.A.; Toramae, J. Statistical optimization of adsorption processes for removal of 2,4-dichlorophenol by activated carbon derived from oil palm empty fruit bunches. J. Environ. Sci 2007, 19, 674–677. [Google Scholar]
- Calace, N.; Nardi, E.; Petronio, B.M.; Pietroletti, M. Adsorption of phenols by papermill sludges. Environ. Pollut 2002, 118, 315–319. [Google Scholar]
- Xiaoli, C.; Youcai, Z. Adsorption of phenolic compound by aged-refuse. J. Hazard. Mater. B 2006, 137, 410–417. [Google Scholar]
- Denizli, A.; Okan, G.; Ucar, M. Dye-affinity microbeads for removal of phenols and nitrophenols from aquatic systems. J. Appl. Polym. Sci 2002, 83, 2411–2418. [Google Scholar]
- Jain, A.K.; Gupta, V.K.; Jain, S.; Has, S. Removal of chlorophenols using industrial wastes. Environ. Sci. Technol 2004, 38, 1195–1200. [Google Scholar]
- Sreenivasan, K. Synthesis and evaluation of a beta-cyclodextrin-based molecularly imprinted copolymer. J. Appl. Polym. Sci 1998, 70, 15–18. [Google Scholar]
- Sreenivasan, K. Grafting of a β-cyclodextrin-modified 2-hydroxyethyl methacrylate onto polyurethane. J. Appl. Polym. Sci 1996, 60, 2245–2249. [Google Scholar]
- Liu, N.; Pan, J.Q.; Lau, W.W.Y. Preparations and properties of new monomeric light stabilizers. Polym. Degrad. Stable 1999, 63, 71–77. [Google Scholar]
- Zakaria, N.D.; Yusof, N.A.; Haron, J.; Abdullah, A.H. Synthesis and evaluation of a molecularly imprinted polymer for 2,4-dinitrophenol. Int. J. Mol. Sci 2009, 10, 354–365. [Google Scholar]









| Isotherm Constants | Temperature (K) | ||
|---|---|---|---|
| 298 | 318 | 338 | |
| Langmuir | |||
| Q max (mg·g−1) | −90.909 | −125 | −90.909 |
| b (L·mg−1) | −0.004 | −0.003 | −0.004 |
| RL | 1.65 | 1.42 | 1.59 |
| R2 | 0.541 | 0.557 | 0.512 |
| Freundlich | |||
| KF (mg·g−1(L·mg−1)1/n) | 2.244 | 0.475 | 0.404 |
| n | 1.025 | 1.009 | 0.975 |
| 1/n | 0.976 | 0.991 | 1.026 |
| R2 | 0.999 | 0.995 | 0.999 |
| Temkin | |||
| KT (L·mg−1) | 0.071 | 0.071 | 0.071 |
| B | 20.18 | 19.6 | 18.54 |
| R2 | 0.962 | 0.948 | 0.967 |
| Dubinin-Radushkevich | |||
| KDR (mol2 kJ2) | 0.023 | 0.024 | 0.021 |
| E (kJ·mol−1) | 4.662 | 4.564 | 4.879 |
| R2 | 0.981 | 0.992 | 0.978 |
| Kinetic Model | Constants | MIP MAA-βCD |
|---|---|---|
| Pseudo-first-order model | qe, experimental (mg·g−1) | 4.585 |
| qe, calculated (mg·g−1) | 0.561 | |
| k1 (min−1) | 0.016 | |
| Δq (%) | 43.882 | |
| Relative error (%) | 87.764 | |
| R2 | 0.67 | |
| Pseudo-second-order model | qe, experimental (mg·g−1) | 4.585 |
| qe, calculated (mg·g−1) | 4.587 | |
| h (mg·g−1 min ) | 4.367 | |
| k2 (g·mg−1 min) | 0.207 | |
| Δq (%) | 0.017 | |
| Relative error (%) | 0.046 | |
| R2 | 0.999 | |
| Elovich model | qe, experimental (mg·g−1) | 4.585 |
| qe, calculated (mg·g−1) | 4.243 | |
| B (g·mg−1) | 1.799 | |
| α (mg·g−1·min−1) | 25.498 | |
| Δq (%) | 3.725 | |
| Relative error (%) | 7.451 | |
| R2 | 0.886 | |
| Intraparticle diffusion | qe, experimental (mg·g−1) | 4.585 |
| C (mg·g−1) | 3.288 | |
| ki (mg·g−1·min1/2) | 0.117 | |
| Δq (%) | 12.651 | |
| Relative error (%) | 28.288 | |
| R2 | 0.525 | |
| External Diffusion | kext (min−1) | 0.003 |
| C | −1.136 | |
| R2 | 0.459 | |
| Sorbent | Thermodynamic Parameters | |||
|---|---|---|---|---|
| ΔH° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | T (K) | ΔG° (kJ·mol−1) | |
| MIP MAA-βCD | −32.09 | 11.64 | 298 | −3.501 |
| 318 | −3.734 | |||
| 338 | −3.966 | |||
| Phenols | MIP MAA-βCD | NIP MAA-βCD | k′ | ||
|---|---|---|---|---|---|
| Kd (mg·g−1) | k | Kd (mg·g−1) | k | ||
| 2,4-DCP | 5.19 | 2.76 | |||
| 4-CMP | 2.54 | 2.04 | 2.34 | 1.18 | 1.73 |
| 2-CP | 1.77 | 2.93 | 1.94 | 1.42 | 2.06 |
| 2-NP | 1.9 | 2.73 | 1.79 | 1.54 | 1.77 |
| 4-NP | 2.65 | 1.96 | 2.25 | 1.23 | 1.59 |
| Adsorbent | Adsorption Capacity, qe (mg·g−1) | Reference |
|---|---|---|
| MIP MAA-βCD | 45.67 | This study |
| Maize cob carbon | 17.94 | [59] |
| Palm pith carbon | 19.16 | [60] |
| Oil palm empty fruit bunch carbon | 27.25 | [61] |
| Paper mill sludge | 4.49 | [62] |
| Aged refuse in biofilter | 1.53 | [63] |
| Poly HEMA microbead | 16.1 | [64] |
| Blast furnace sludge | 29.1 | [65] |
| β-CD Attalpugite composites | 19.04 | [36] |
| β-CD epichlorohydrin polymer | 15.7 | [39] |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Surikumaran, H.; Mohamad, S.; Sarih, N.M. Molecular Imprinted Polymer of Methacrylic Acid Functionalised ?-Cyclodextrin for Selective Removal of 2,4-Dichlorophenol. Int. J. Mol. Sci. 2014, 15, 6111-6136. https://doi.org/10.3390/ijms15046111
Surikumaran H, Mohamad S, Sarih NM. Molecular Imprinted Polymer of Methacrylic Acid Functionalised ?-Cyclodextrin for Selective Removal of 2,4-Dichlorophenol. International Journal of Molecular Sciences. 2014; 15(4):6111-6136. https://doi.org/10.3390/ijms15046111
Chicago/Turabian StyleSurikumaran, Hemavathy, Sharifah Mohamad, and Norazilawati Muhamad Sarih. 2014. "Molecular Imprinted Polymer of Methacrylic Acid Functionalised ?-Cyclodextrin for Selective Removal of 2,4-Dichlorophenol" International Journal of Molecular Sciences 15, no. 4: 6111-6136. https://doi.org/10.3390/ijms15046111
APA StyleSurikumaran, H., Mohamad, S., & Sarih, N. M. (2014). Molecular Imprinted Polymer of Methacrylic Acid Functionalised ?-Cyclodextrin for Selective Removal of 2,4-Dichlorophenol. International Journal of Molecular Sciences, 15(4), 6111-6136. https://doi.org/10.3390/ijms15046111




