Vitamin D: Link between Osteoporosis, Obesity, and Diabetes?
Abstract
:1. Introduction
2. Methodology
3. Vitamin D
4. Vitamin D and Osteoporosis
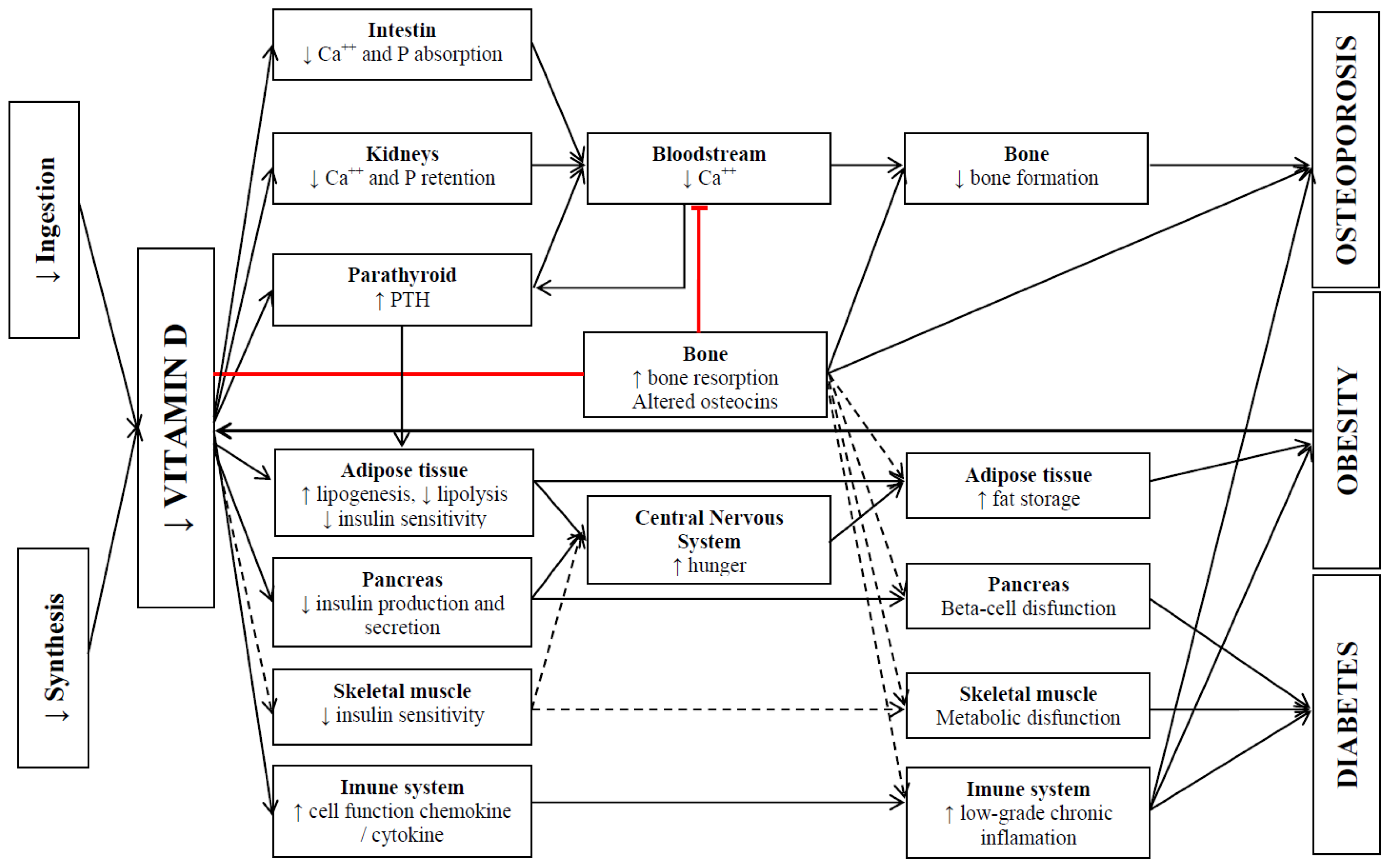
4.1. Mechanisms of Action
4.2. Scientific Evidences
4.2.1. Vitamin D and BMD
4.2.2. Vitamin D and Falls/Fractures
5. Vitamin D, Obesity, and T2DM
5.1. Mechanisms of Action
5.2. Scientific Evidences
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lips, P.; Schoor, N.M.V. The effect of vitamin D on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab 2011, 25, 585–591. [Google Scholar]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.M.; Murad, H.; Kovacs, C.S. The nonskeletal effects of vitamin D: An endocrine society scientific statement. Endocr. Rev 2012, 33, 456–492. [Google Scholar]
- Gomez-Ambrosi, J.; Rodrıguez, A.; Catalan, V.; Fruhbeck, G. The bone-adipose axis in obesity and weight loss. Obes. Surg 2008, 18, 1134–1143. [Google Scholar]
- Migliaccio, S.; Greco, E.A.; Fornari, R.; Donini, L.M.; Lenzi, A. Is obesity in women protective against osteoporosis? Diabetes Metab. Syndr. Obes 2011, 4, 273–282. [Google Scholar]
- Ross, A.C.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S.; et al. Institute of medicine (US) committee to review dietary reference intakes for vitamin D and calcium. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Valle, H.B.D., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab 2011, 96, 1911–1930. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab 2012, 97, 1153–1158. [Google Scholar]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int 2013, 92, 77–98. [Google Scholar]
- Ashwell, M.; Stone, E.M.; Stolte, H.; Cashman, K.D.; Macdonald, H.; Lanham-New, S.; Hiom, S.; Webb, A.; Frase, D. UK food standards agency workshop report: An investigation of the relative contributions of diet and sunlight to vitamin D status. Br. J. Nutr 2010, 104, 603–611. [Google Scholar]
- Tsiaras, W.G.; Weinstoc, M.A. Factors influencing vitamin D status. Acta Derm. Venereol 2011, 91, 115–124. [Google Scholar]
- Bens, G. Sunscreens. Adv. Exp. Med. Biol 2008, 624, 137–161. [Google Scholar]
- Blum, M.; Dolnikowski, G.; Seyoum, E.; Harris, S.S.; Booth, S.L.; Peterson, J.; Saltzman, E.; Dawson-Hughes, B. Vitamin D(3) in fat tissue. Endocrine 2008, 33, 90–94. [Google Scholar]
- Hanley, D.A.; Cranney, A.; Jones, G.; Whiting, S.J.; Leslie, W.D.; Cole, D.E.C.; Atkinson, S.A.; Josse, R.G.; Feldman, S.; Kline, G.A.; et al. Vitamin D in adult health and disease: A review and guideline statement from Osteoporosis Canada. Can. Med. Assoc. J 2010, 182, 610–618. [Google Scholar]
- Holick, M.F. The role of vitamin D for bone health and fracture prevention. Curr. Osteoporos. Rep 2006, 4, 96–102. [Google Scholar]
- Souberbielle, J.C.; Body, J.J.; Lappe, J.M.; Plebani, M.; Shoenfeld, Y.; Wang, T.J.; Bischoff-Ferrari, H.A.; Cavalier, E.; Ebeling, P.R.; Fardellone, P.; et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev 2010, 9, 709–715. [Google Scholar]
- Bischoff-Ferrari, H.A.; Shao, A.; Dawson-Hughes, B.; Hathcock, J.; Giovannucci, E.; Willett, W.C. Benefit-Risk assessment of vitamin D supplementation. Osteoporos. Int 2010, 21, 1121–1132. [Google Scholar]
- Cunningham, J.; Locatelli, F.; Rodriguez, M. Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin. J. Am. Soc. Nephrol 2011, 6, 913–921. [Google Scholar]
- Lamprecht, A.S.; Lipkin, M. Chemoprevention of colon cancer by calcium, vitamin D and folate: Molecular mechanisms. Nat. Rev. Cancer 2003, 3, 601–614. [Google Scholar]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol 2010, 5, 23–30. [Google Scholar]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol 2006, 92, 4–8. [Google Scholar]
- Yasuda, H.; Higashio, K.; Suda, T. Vitamin D and osteoclastogenesis. In Vitamin D; Feldman, D., Pike, J.W., Glorieux, F.H., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2005; Volume 2, pp. 665–685. [Google Scholar]
- Lisse, T.S.; Chun, R.F.; Rieger, S.; Adams, J.S.; Hewison, M. Vitamin D activation of functionally distinct regulatory miRNAs in primary human osteoblasts. J. Bone Miner. Res 2013, 28, 1478–1488. [Google Scholar]
- Kogawa, M.; Anderson, P.H.; Findlaya, D.M.; Morris, H.A.; Atkins, G.J. The metabolism of 25-(OH) vitamin D3 by osteoclasts and their precursors regulates the differentiation of osteoclasts. J. Steroid Biochem. Mol. Biol 2010, 121, 277–280. [Google Scholar]
- Diamond, T.; Wong, Y.K.; Golombick, T. Effect of oral cholecalciferol 2000 vs. 5000 IU on serum vitamin D, PTH, bone and muscle strength in patients with vitamin D deficiency. Osteoporos. Int 2013, 24, 1101–1105. [Google Scholar]
- Martin, T.J.; Sims, N.A. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol. Med 2005, 11, 76–81. [Google Scholar]
- Zhu, K.; Devine, A.; Dick, I.M.; Wilson, S.G.; Prince, R.L. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: A five-year randomized controlled trial. J. Clin. Endocrinol. Metab 2008, 93, 743–749. [Google Scholar]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Dawson-Hughes, B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: A population-based study of younger and older adults. Am. J. Med 2004, 116, 634–639. [Google Scholar]
- Kuchuk, N.O.; Pluijm, S.M.; van Schoor, N.M.; Looman, C.W.; Smit, J.H.; Lips, P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J. Clin. Endocrinol. Metable 2009, 94, 1244–1250. [Google Scholar]
- Kuchuk, N.O.; van Schoor, N.M.; Pluijm, S.M.; Chines, A.; Lips, P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: Global perspective. J. Bone Miner. Res 2009, 24, 693–701. [Google Scholar]
- Van Schoor, N.M.; Visser, M.; Pluijm, S.M.; Kuchuk, N.; Smit, J.H.; Lips, P. Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone 2008, 42, 260–266. [Google Scholar]
- Larrosa, M.; Gomez, A.; Casado, E.; Moreno, M.; Vázquez, I.; Orellana, C.; Berlanga, E.; Ramon, J.; Gratacos, J. Hypovitaminosis D as a risk factor of hip fracture severity. Osteoporos. Int 2012, 23, 607–661. [Google Scholar]
- Holick, M.F.; Siris, E.S.; Binkley, N.; Beard, M.K.; Khan, A.; Katzer, J.T.; Petruschke, R.A.; Chen, E.; de Papp, A.E. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J. Clin. Endocrinol. Metab 2005, 8, 3215–3224. [Google Scholar]
- Harwood, R.H.; Sahota, O.; Gaynor, K.; Masud, T.; Hosking, D.J. A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: The Nottingham Neck of Femur (NONOF) study. Age Ageing 2004, 33, 45–51. [Google Scholar]
- Jackson, R.D.; LaCroix, A.Z.; Gass, M.; Wallace, R.B.; Robbins, J.; Lewis, C.E.; Bassford, T.; Beresford, S.A.A.; Black, H.R.; Blanchette, P.; et al. Calcium plus vitamin D supplementation and the risk of fractures. N. Engl. J. Med 2006, 354, 669–683. [Google Scholar]
- Karkkainen, M.; Tuppurainen, M.; Salovaara, K.; Sandini, L.; Rikkonen, T.; Sirola, J.; Honkanen, R.; Jurvelin, J.; Alhava, E.; Kröger, H. Effect of calcium and vitamin D supplementation on bone mineral density in women aged 65–71 years: A 3-year randomized population-based trial (OSTPRE-FPS). Osteoporos. Int 2010, 21, 2047–2055. [Google Scholar]
- Moschonis, G.; Katsaroli, I.; Lyritis, G.P.; Manios, Y. The effects of a 30-month dietary intervention on bone mineral density: The postmenopausal health study. Br. J. Nutr 2010, 104, 100–107. [Google Scholar]
- Jorde, R.; Sneve, M.; Torjesen, P.A.; Figenschau, Y.; Hansen, J-B.; Grimnes, G. No significant effect on bone mineral density by high doses of vitamin D3 given to overweight subjects for one year. Nutr. J 2010, 9, 1. [Google Scholar] [CrossRef]
- Islam, M.Z.; Shamim, A.; Viljakainen, H.T.; Akhtaruzzaman, M.; Jehan, A.H.; Khan, H.U.; Al-Arif, F.A.; Lamberg-Allardt, C. Effect of vitamin D, calcium and multiple micronutrient supplementation on vitamin D and bone status in Bangladeshi premenopausal garment factory workers with hypovitaminosis D: A double-blinded, randomised, placebo-controlled 1-year intervention. Br. J. Nutr 2010, 104, 241–247. [Google Scholar]
- Rastelli, A.L.; Taylor, M.E.; Gao, F.; Armamento-Villareal, R.; Jamalabadi-Majidi, S.; Napoli, N.; Ellis, M.J. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): A phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res. Treat 2011, 129, 107–116. [Google Scholar]
- Steffensen, L.H.; Jorgensen, L.; Straume, B.; Mellgren, S.I.; Kampman, M.T. Can vitamin D(3) supplementation prevent bone loss in persons with MS? A placebo-controlled trial. J. Neurol 2011, 258, 1624–1631. [Google Scholar]
- Verschueren, S.M.P.; Bogaerts, A.; Delecluse, C.; Claessens, A.L.; Haentjens, P.; Vanderschueren, D.; Boonen, S. The effects of whole-body vibration training and vitamin D supplementation on muscle strength, muscle mass, and bone density in institutionalized elderly women: A 6-month randomized, controlled trial. J. Bone Miner. Res 2011, 26, 42–49. [Google Scholar]
- Grimnes, G.; Joakimsen, R.; Figenschau, Y.; Torjesen, P.A.; Almas, B.; Jorde, R. The effect of high-dose vitamin D on bone mineral density and bone turnover markers in postmenopausal women with low bone mass-a randomized controlled 1-year trial. Osteoporos. Int 2012, 23, 201–211. [Google Scholar]
- Nieves, J.; Cosman, F.; Grubert, E.; Ambrose, B.; Ralston, S.; Lindsay, R. Skeletal effects of vitamin D supplementation in postmenopausal black women. Calcif. Tissue Int 2012, 91, 316–324. [Google Scholar]
- Macdonald, H.M.; Wood, A.D.; Aucott, L.S.; Black, A.J.; Fraser, W.D.; Mavroeidi, A.; Reid, D.M.; Secombes, K.R.; Simpson, W.G.; Thies, F. Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: A 1 year double-blind RCT in postmenopausal women. J. Bone Miner. Res 2013, 28, 2202–2213. [Google Scholar]
- Wamberg, L.; Pedersen, S.B.; Richelsen, B.; Rejnmark, L. The effect of high-dose vitamin D supplementation on calciotropic hormones and bone mineral density in obese subjects with low levels of circulating 25-hydroxyvitamin D: Results from a randomized controlled study. Calcif. Tissue Int 2013, 93, 69–77. [Google Scholar]
- Lenchik, L.; Kiebzak, G.M.; Blunt, B.A. What is the role of serial bone mineral density measurements in patient management? J. Clin. Densitom 2002, 5, 29–38. [Google Scholar]
- Trivedi, D.; Doll, R.; Khaw, K. Effect of four monthly oral vitamin D supplementation on fractures and mortality in men and women living in the community: Randomized double blind controlled trial. BMJ 2003, 326, 469–475. [Google Scholar]
- Larsen, E.R.; Mosekilde, L.; Foldspang, A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: A pragmatic population-based 3-year intervention study. J. Bone Miner. Res 2004, 19, 370–378. [Google Scholar]
- Grant, A.M.; Avenell, A.; Campbell, M.K.; McDonald, A.M.; MacLennan, G.S.; McPherson, G.C.; Anderson, F.H.; Cooper, C.; Francis, R.M.; Donaldson, C.; et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised evaluation of Calcium or vitamin D, RECORD): A randomised placebo-controlled trial. Lancet 2005, 365, 1621–1628. [Google Scholar]
- Porthouse, J.; Cockayne, S.; King, C.; Saxon, L.; Steele, E.; Aspray, T.; Baverstock, M.; Birks, Y.; Dumville, J.; Francis, R.; et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ 2005, 330, 1003. [Google Scholar]
- Flicker, L.; MacInnis, R.J.; Stein, M.S.; Scherer, S.C.; Mead, K.E.; Nowson, C.A.; Thomas, J.; Lowndes, C.; Hopper, J.L.; Wark, J.D. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J. Am. Geriatr. Soc 2005, 53, 1881–1888. [Google Scholar]
- Lyons, R.A.; Johansen, A.; Brophy, S.; Newcombe, R.G.; Phillips, C.J.; Lervy, B.; Evans, R.; Wareham, K.; Stone, M.D. Preventing fractures among older people living in institutional care: A pragmatic randomised double blind placebo controlled trial of vitamin D supplementation. Osteoporos. Int 2007, 18, 811–818. [Google Scholar]
- Smith, H.; Anderson, F.; Raphael, H.; Maslin, P.; Crozier, S.; Cooper, C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women: a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford) 2007, 46, 1852–1857. [Google Scholar]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Suppan, K.; Fahrleitner-Pammer, A.; Dobnig, H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos. Int 2009, 20, 315–322. [Google Scholar]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar]
- Salovaara, K.; Tuppurainen, M.; Karkkainen, M.; Rikkonen, T.; Sandini, L.; Sirola, J.; Honkanen, R.; Alhava, E.; Kröger, H. Effect of vitamin D(3) and calcium on fracture risk in 65- to 71-year-old women: A population-based 3-year randomized, controlled trial: The OSTPRE-FPS. J. Bone Miner. Res 2010, 25, 1487–1495. [Google Scholar]
- Bakhtiyarova, S.; Lesnyak, O.; Kyznesova, N.; Blankenstein, M.A.; Lips, P. Vitamin D status among patients with hip fracture and elderly control subjects in Yekaterinburg, Russia. Osteoporos. Int 2006, 17, 441–446. [Google Scholar]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Orav, E.J.; Lips, P.; Meunier, P.J.; Flicker, L.; Wark, J.; Jackson, R.D.; Cauley, J.A.; Meyer, H.E.; et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N. Engl. J. Med 2012, 367, 40–49. [Google Scholar]
- Lamendola, C.A.; Ariel, D.; Feldman, D.; Reaven, G.M. Relations between obesity, insulin resistance, and 25-hydroxyvitamin D. Am. J. Clin. Nutr 2012, 95, 1055–1059. [Google Scholar]
- Norman, A.W. Minireview: Vitamin D receptor: New assignments for an already busy receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys 2012, 523, 123–133. [Google Scholar]
- Bland, R.; Markovic, D.; Hills, C.E.; Hughes, S.V.; Chan, S.L.; Squires, P.E.; Hewison, M. Expression of 25-hydroxyvitamin D3-1α-hydroxylase in pancreatic islets. J. Steroid Biochem. Mol. Biol 2004, 89, 121–125. [Google Scholar]
- Ding, C.; Gao, D.; Wilding, J.; Trayhurn, P.; Bing, C. Vitamin D signalling in adipose tissue. Br. J. Nutr 2012, 108, 1915–192. [Google Scholar]
- Maestro, B.; Davila, N.; Carranza, M.C.; Calle, C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J. Steroid Biochem. Mol. Biol 2003, 84, 223–230. [Google Scholar]
- Wolden-Kirk, H.; Overbergh, L.; Christesen, H.T.; Brusgaard, K.; Mathieu, C. Vitamin D and diabetes: Its importance for beta cell and immune function. Mol. Cell. Endocrinol 2011, 347, 106–120. [Google Scholar]
- Alvarez, J.A.; Ashraf, A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int. J. Endocrinol 2010. [Google Scholar] [CrossRef]
- Soares, M.J.; Murhadi, L.L.; Kurpad, A.V.; She, Chan; Ping-Delfos, W.L.; Piers, L.S. Mechanistic roles for calcium and vitamin D in the regulation of body weight. Obes. Rev 2012, 13, 592–605. [Google Scholar]
- De Paula, F.J.; Dick-de-Paula, I.; Bornstein, S.; Rostama, B.; Le, P.; Lotinun, S.; Baron, R.; Rosen, C.J. VDR haploinsufficiency impacts body composition and skeletal acquisition in a gender specific manner. Calcif. Tissue Int 2011, 89, 179–191. [Google Scholar]
- Maestro, B.; Molero, S.; Bajo, S.; Dávila, N.; Calle, C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D3. Cell Biochem. Funct 2002, 20, 227–232. [Google Scholar]
- Dunlop, T.W.; Väisänen, S.; Frank, C.; Molnár, F.; Sinkkonen, L.; Carlberg, C.; Karn, J. The human peroxisome proliferator-activated receptor δ gene is a primary target of 1α, 25-dihydroxyvitamin D3 and its nuclear receptor. J. Mol. Biol 2005, 349, 248–260. [Google Scholar]
- Maestro, B.; Campion, J.; Davila, N.; Calle, C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr. J 2000, 47, 383–391. [Google Scholar]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med 2007, 357, 266–281. [Google Scholar]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bi-directional mendelian randomization analysis of multiple cohorts. PLoS Med 2013, 10, 1–13. [Google Scholar]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar]
- Gómez-Ambrosi, J.; Catalán, V.; Ramírez, B.; Rodríguez, A.; Colina, I.; Silva, C.; Rotellar, F.; Mugueta, C.; Gil, M.J.; Cienfuegos, J.A.; et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J. Clin. Endocrinol. Metab 2007, 92, 3719–3727. [Google Scholar]
- Nomiyama, T.; Perez-Tilve, D.; Ogawa, D.; Gizard, F.; Zhao, Y.; Heywood, E.B.; Jones, K.L.; Kawamori, R.; Cassis, L.A.; Tschöp, M.H.; et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Investig 2007, 117, 2877–2888. [Google Scholar]
- Fukumoto, S.; Martin, T.J. Bone as an endocrine organ. Trends Endocrin. Met 2009, 20, 230–236. [Google Scholar]
- Almerighi, C.; Sinistro, A.; Cavazza, A.; Ciaprini, C.; Rocchi, G.; Bergamini, A. 1α,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine 2009, 45, 190–197. [Google Scholar]
- Eftekharian, M.M.; Zarnani, A.H.; Moazzeni, S.M. In vivo effects of calcitriol on phenotypic and functional properties of dendritic cells. Iran J. Immunol 2010, 7, 74–82. [Google Scholar]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol 2009, 183, 5458–5467. [Google Scholar]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol 2007, 179, 1634–1647. [Google Scholar]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C.; Vitamin, D. Modulator of the immune system. Curr. Opin. Pharmacol 2010, 10, 482–496. [Google Scholar]
- Ducloux, R.; Nobécourt, E.; Chevallier, J.M.; Ducloux, H.; Elian, N.; Altman, J.J. Vitamin D deficiency before bariatric surgery: Should supplement intake be routinely prescribed? Obes. Surg 2011, 21, 556–560. [Google Scholar]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab 2007, 92, 2017–2029. [Google Scholar]
- De Boer, I.H.; Tinker, L.F.; Connelly, S.; Curb, J.D.; Howard, B.V.; Kestenbaum, B.; Larson, J.C.; Manson, J.E.; Margolis, K.L.; Siscovick, D.S.; et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care 2008, 31, 701–707. [Google Scholar]
- Aloia, J.F. African Americans, 25-hydroxyvitamin D, and osteoporosis: A paradox. Am. J. Clin. Nutr 2008, 88, 545–550. [Google Scholar]
- Devaraj, S.; Jialal, G.; Cook, T.; Siegel, D.; Jialal, I. Low vitamin D levels in Northern American adults with the metabolic syndrome. Horm. Metab. Res 2011, 43, 72–74. [Google Scholar]
- Elsammak, M.Y.; Al-Wosaibi, A.A.; Al-Howeish, A.; Alsaeed, J. Vitamin D deficiency in Saudi Arabs. Horm. Metab 2010, 42, 364–368. [Google Scholar]
- Binkley, N.; Novotny, R.; Krueger, D.; Kawahara, T.; Daida, Y.G.; Lensmeyer, G.; Hollis, B.W.; Drezner, M.K. Low vitamin D status despite abundant sun exposure. J. Clin. Endocrinol. Metab 2007, 92, 2130–2135. [Google Scholar]
- Snijder, M.B.; van Dam, R.M.; Visser, M.; Deeg, D.J.; Dekker, J.M.; Bouter, L.M.; Seidell, J.C.; Lips, P. Adiposity in relation to vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J. Clin. Endocrinol. Metab 2005, 90, 4119–4123. [Google Scholar]
- Shankar, A.; Sabanayagam, C.; Kalidindi, S. Serum 25-hydroxyvitamin D levels and prediabetes among subjects free of diabetes. Diabetes Care 2011, 34, 1114–1119. [Google Scholar]
- Pittas, A.G.; Chung, M.; Trikalinos, T.; Mitri, J.; Brendel, M.; Patel, K.; Lichtenstein, A.H.; Lau, J.; Balk, E.M. Systematic review: Vitamin D and cardiometabolic outcomes. Ann. Intern. Med 2010, 152, 307–314. [Google Scholar]
- Jorde, R.; Sneve, M.; Emaus, N.; Figenschau, Y.; Grimnes, G. Cross-sectional and longitudinal relation betweenserum 25-hydroxyvitamin D and body mass index: The Tromso study. Eur. J. Nutr 2010, 49, 401–407. [Google Scholar]
- Lagunova, Z.; Porojnicu, A.C.; Lindberg, F.A.; Aksnes, L.; Moan, J. Vitamin D status in Norwegian children and adolescents with excess body weight. Pediatr. Diabetes 2011, 12, 120–126. [Google Scholar]
- Nunlee-Bland, G.; Gambhir, K.; Abrams, C.; Abdul, M.; Vahedi, M.; Odonkor, W. Vitamin D deficiency and insulin resistance in obese African-American adolescents. J. Pediatr. Endocrinol. Metab 2011, 24, 29–33. [Google Scholar]
- Rajakumar, K.; de las Heras, J.; Chen, T.C.; Lee, S.; Holick, M.F.; Arslanian, S.A. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J. Clin. Endocrinol. Metab 2011, 96, 1560–1567. [Google Scholar]
- Muscogiuri, G.; Sorice, G.P.; Prioletta, A.; Pollicola, C.; della Casa, S.; Pontecorvi, A.; Giaccari, A. 25-hydroxyvitamin D concentration correlates with insulin sensitivity and BMI in obesity. Obesity 2010, 18, 1906–1910. [Google Scholar]
- Gulseth, H.L.; Gjelstad, I.M.; Tierney, A.C.; Lovegrove, J.A.; Defoort, C.; Blaak, E.E.; Lopez-Miranda, J.; Kiec-Wilk, B.; Risérus, U.; Roche, H.M.; et al. Serum vitamin D concentration does not predict insulin action or secretion in European subjects with the metabolic syndrome. Diabetes Care 2010, 33, 923–925. [Google Scholar]
- Del Gobbo, L.C.; Song, Y.; Dannenbaum, D.A.; Dewailly, E.; Egeland, G.M. Serum 25-hydroxyvitamin D is not associated with insulin resistance or beta cell function in Canadian Cree. J. Nutr 2011, 141, 290–295. [Google Scholar]
- Chacko, S.A.; Song, Y.; Manson, J.E.; van Horn, L.; Eaton, C.; Martin, L.W.; McTiernan, A.; Curb, J.D.; Wylie-Rosett, J.; Phillips, L.S.; et al. Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in postmenopausal women. Am. J. Clin. Nutr 2011, 94, 209–217. [Google Scholar]
- Rosenblum, J.L.; Castro, V.M.; Moore, C.E.; Kaplan, L.M. Calcium and vitamin D supplementation is associated with decreased abdominal visceral adipose tissue in overweight and obese adults. Am. J. Clin. Nutr 2012, 95, 101–108. [Google Scholar]
- Forsythe, L.K.; Livingstone, M.B.; Barnes, M.S.; Horigan, G.; McSorley, E.M.; Bonham, M.P.; Magee, P.J.; Hill, T.R.; Lucey, A.J.; Cashman, K.D.; et al. Effect of adiposity on vitamin D status and the 25-hydroxycholecalciferol response to supplementation in healthy young and older Irish adults. Br. J. Nutr 2012, 107, 126–134. [Google Scholar]
- Zhou, J.; Zhao, L.J.; Watson, P.; Zhang, Q.; Lappe, J.M. The effect of calcium and vitamin D supplementation on obesity in postmenopausal women: secondary analysis for a large-scale, placebo controlled, double-blind, 4-year longitudinal clinical trial. Nutr. Metab 2010, 23, 7–62. [Google Scholar]
- Major, G.C.; Alarie, F.; Doré, J.; Phouttama, S.; Tremblay, A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am. J. Clin. Nutr 2007, 85, 54–59. [Google Scholar]
- Zittermann, A.; Frisch, S.; Berthold, H.K.; Götting, C.; Kuhn, J.; Kleesiek, K.; Stehle, P.; Koertke, H.; Koerfer, R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am. J. Clin. Nutr 2009, 89, 1321–1327. [Google Scholar]
- Nagpal, J.; Pande, J.N.; Bhartia, A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabetes Med 2009, 26, 19–27. [Google Scholar]
- Sneve, M.; Figenschau, Y.; Jorde, R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur. J. Endocrinol 2008, 159, 675–684. [Google Scholar]

| Study | n (gender) y, duration | Vit D suppl. | Ca++ suppl. | Design | Final serum 25(OH)D3 (nmol/L) | Bone outcomes |
|---|---|---|---|---|---|---|
| Trivedi et al. [47] | 2686 (649F, 2037M) 65–85 y, 5 years | 100,000 IU/4 months | - |
|
|
|
| Harwood et al. [33] | 150 (F) 67–92 y, One year | 800 IU/day; 300,000/year | 1000 mg/day |
|
|
|
| Larsen et al. [48] | 9605 (5771F, 3834M) 66–103 y, 3 years | 400 IU/day | 1000 mg/day |
|
| |
| Grant et al. [49] | 5292 (4481F, 811M) 70 y or older, 2–5.2 years | 800 IU/day | 1000 mg/day |
| - |
|
| Porthouse et al. [50] | 3314 (F) 70 y or older, 1.5–3.5 years | 800 IU/day | 1000 mg/day |
| - |
|
| Flicker et al. [51] | 625 (593F, 32M) 2 years | 10,000 IU/week (1); 1000 IU/day (2) | 600 mg/day |
| - |
|
| Jackson et al. [34] | 36,282 (F) 50–79 y, 7 years | 400 UI/day | 1000 mg/day |
| - |
|
| Lyons et al. [52] | 3440 (2624F, 816M) 62–107 y, 3 years | 100,000 IU/4 months | - |
|
|
|
| Smith et al. [53] | 9440 (5086F, 4354M) 75 y or older, 3 years | 300,000 IU/year | - |
| - |
|
| Zhu et al. [26] | 120 (F) 70–80 y, 5 years | 1000 IU/day | 1200 mg/day |
|
|
|
| Pfeifer et al. [54] | 242 (191F, 51M) 70 y or older, One year | 800 IU/day | 1000 mg/day |
|
|
|
| Kärkkäinen et al. [35] | 593 (F) 66–71 y, 3 years | 800 IU/day | 1000 mg/day |
|
|
|
| Moschonis et al. [36] | 66 (F) 55–65 y, 2.5 years | 300 IU/day (1) 900 IU (2) | 1200 mg/day |
| - |
|
| Jorde et al. [37] | 421 (265F, 156M) 21–70 y, One year | 40,000 IU/week (1) 20,000 IU/week (2) | 500 mg/day |
|
|
|
| Sanders et al. [55] | 2256 (F) 70 y or older, 3–5 years | 500,000 IU/year | - |
|
|
|
| Salovaara et al. [56] | 3432 (F) 65–71 y, 3 years | 800 IU/day | 1000 mg/day |
|
|
|
| Islam et al. [38] | 200 (F) 16–36 y, One year | 400 IU/day | 600 mg/day |
|
|
|
| Rastelli et al. [39] | 60 (F) Mean values of 60 and 63 y 6 months | 400 IU/day + 50,000 IU/weekly and then monthly | 1000 mg/day |
|
|
|
| Steffensen et al. [40] | 71 (F, M) 18–50 y, 2 years | 20,000 IU/week | 500 mg/day |
|
|
|
| Verschueren et al. [41] | 113 (F) 70 y or older, 6 months | 880 IU/day (1) 1600 IU/day (2) | 1000 mg/day |
|
|
|
| Grimnes et al. [42] | 297 (F) 50–80 y, One year | 6500 IU/day (1) 800 IU/day (2) | 1000 mg/day |
|
|
|
| Nieves et al. [43] | 103 (F) Mean values of 62.3 and 61.2 y, 2 years | 1000 IU/day | 1000 mg/day |
|
|
|
| Macdonald et al. [44] | 305 (F) 60–70 y, One year | 400 IU/day (1) 1000 IU/day (2) | - |
|
|
|
| Wamberg et al. [45] | 52 (F, M) 18–50 y, 6 months | 7000 IU/day | - |
|
|
|
| Study | n (gender) y, duration | Vit D suppl. | Ca++ suppl. | Design | Outcomes |
|---|---|---|---|---|---|
| Major et al. [104] | 63 (F) ~42.6 y, 15 weeks | 200 IU/day | 600 mg/day |
|
|
| Pittas et al. [84] | 314 (F, M) 65 y or older, 3 years | 700 IU/day | 500 mg/day |
|
|
| de Boer et al. [85] | 33,951 (F) 50–79 y, 7 years | 400 IU/day | 1000 mg/day |
|
|
| Nagpal et al. [106] | 100 (M) 35 y or older, 6 weeks | 360,000 IU/fortnightly | - |
|
|
| Sneve et al. [107] | 445 (F, M) 21–70 y, 12 months | 20,000 IU/twice a week (1) 20,000 IU/once a week (2) | 500 mg/day |
|
|
| Zitterman et al. [105] | 200 (F, M) 18–70 y, 12 months | 3320 IU/day | - |
|
|
| Zhou et al. [103] | 870 (F) 55 y or older, 4 years | 1100 IU/day | 1400–1500 mg/day |
|
|
| Rosenmblum et al. [101] | 171 (F, M) 18–65 y, 16 weeks | 300 IU/day | 1050 mg/day |
|
|
| Forsythe et al. [102] | 212 (F, M) 20–40; >64 y, 22 weeks | 600 IU/day | - |
|
|
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cândido, F.G.; Bressan, J. Vitamin D: Link between Osteoporosis, Obesity, and Diabetes? Int. J. Mol. Sci. 2014, 15, 6569-6591. https://doi.org/10.3390/ijms15046569
Cândido FG, Bressan J. Vitamin D: Link between Osteoporosis, Obesity, and Diabetes? International Journal of Molecular Sciences. 2014; 15(4):6569-6591. https://doi.org/10.3390/ijms15046569
Chicago/Turabian StyleCândido, Flávia Galvão, and Josefina Bressan. 2014. "Vitamin D: Link between Osteoporosis, Obesity, and Diabetes?" International Journal of Molecular Sciences 15, no. 4: 6569-6591. https://doi.org/10.3390/ijms15046569




