Simultaneous Microwave Extraction and Separation of Volatile and Non-Volatile Organic Compounds of Boldo Leaves. From Lab to Industrial Scale
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Conventional Method of Extraction
2.2. Determination of Solid/Liquid Ratio
2.3. Studies Using an Experimental Design
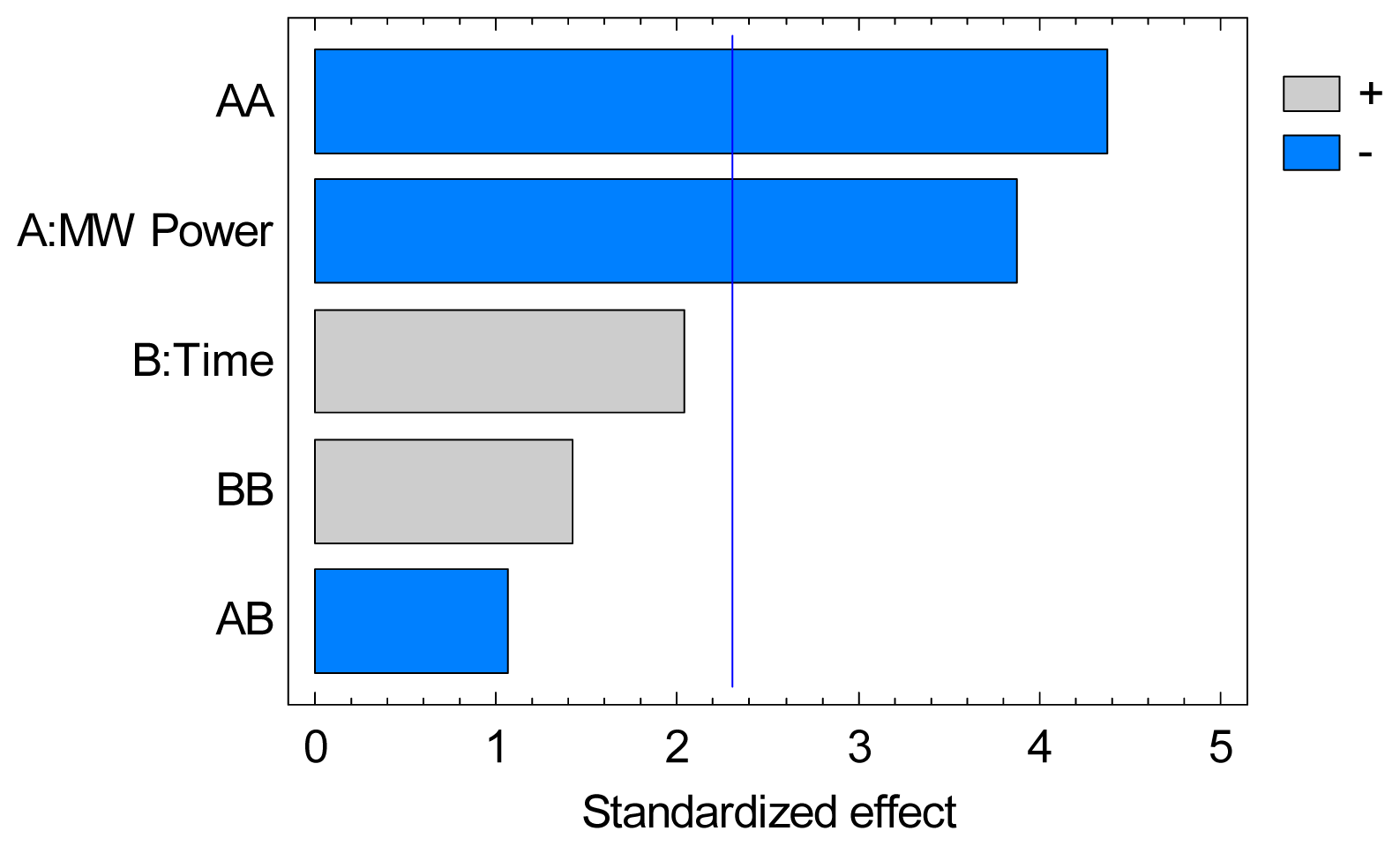
2.3.1. Results for NVOC and VOC Yields
2.3.2. Optimization of Yield of Extraction and Separation of VOC and NVOC
2.4. From Lab Scale to Pilot Scale
2.5. Analysis of the Essential Oil
2.6. Sensorial Analysis
3. Experimental Section
3.1. Plant Material and Chemicals
3.2. Extraction Procedures
3.2.1. Microwave Assisted Extraction (MAE)
3.2.2. Conventional Hydrodistillation (HD)
3.2.3. Scale up Microwave Assisted Extraction
- - The microwaves reach the sensing element of the sensor, producing an electrical noise resulting in a mismeasurement.
- - The microwaves electrically charge the metallic container of the sensor up to very high voltage, producing sparks near to the sensors tip. The temperature can increase locally due to the sparks.
- - The RTD we use is fully embedded in a metallic container (Stainless steel AISI 316) and is a perfect Faraday’s cage. In this way the microwaves do not reach the sensing element at all.
- - The metallic container of the sensor is grounded, the tip is shorter than microwaves wavelength and recessed in a metallic shield of specific length and diameter. This construction is a microwaves filter: the 2450 MHz generated cannot electrically charge the metallic container of the sensor to high voltage. In this way the microwaves do not produce sparks on the sensor surface.
3.3. Analysis of Extracts
3.3.1. Dry Mass Percentage
3.3.2. Isolated Compound Study
3.3.3. Boldine Analysis
3.4. Gas Chromatography and Mass Spectrometry Analysis
3.5. Experimental Design
3.6. Sensorial Analysis
4. Conclusions
Conflicts of Interest
References
- Vogel, H.; González, B.; Razmilic, I. Boldo (Peumus boldus) cultivated under different light conditions, soil humidity and plantation density. Ind. Crops Prod 2011, 34, 1310–1312. [Google Scholar]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Direct identification of phenolic constituents in Boldo Folium (Peumus boldus Mol.) infusions by high-performance liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 443–449. [Google Scholar]
- Schmeda-Hirschmann, G.; Rodriguez, J.A.; Theoduloz, C.; Astudillo, S.L.; Feresin, G.E.; Tapia, A. Free-radical scavengers and antioxidants from Peumus boldus Mol. (“Boldo”). Free Radic. Res 2003, 37, 447–452. [Google Scholar]
- Quezada, N.; Asencio, M.; del Valle, J.M.; Aguilera, J.M.; Gómez, B. Antioxidant activity of crude extract, alkaloid fraction, and flavonoid fraction from boldo (Peumus boldus Molina) leaves. J. Food Sci 2004, 69, C371–C376. [Google Scholar]
- Kringstein, P.; Cederbaum, A.I. Boldine prevents human liver microsomal lipid peroxidation and inactivation of cytochrome P4502E1. Free Radic. Biol. Med 1995, 18, 559–563. [Google Scholar]
- Cederbaum, A.I.; Ukielka, E.K.; Speiskyf, H. Inhibition of rat liver microsomal lipid peroxidation by boldine. Biochem. Pharmacol 1992, 44, 1765–1772. [Google Scholar]
- MacDonald, D.; VanCrey, K.; Harrison, P.; Rangachari, P.K.; Rosenfeld, J.; Warren, C.; Sorger, G. Ascaridole-less infusions of Chenopodium ambrosioides contain a nematocide(s) that is(are) not toxic to mammalian smooth muscle. J. Ethnopharmacol 2004, 92, 215–221. [Google Scholar]
- Karlberg, A.-T.; Magnusson, K.; Nilsson, U. Air oxidation of d-limonene (the citrus solvent) creates potent allergens. Contact Dermat 1992, 26, 332–340. [Google Scholar]
- Rudbäck, J.; Bergström, M.A.; Börje, A.; Nilsson, U.; Karlberg, A.-T. α-Terpinene, an antioxidant in tea tree oil, autoxidizes rapidly to skin allergens on air exposure. Chem. Res. Toxicol 2012, 25, 713–721. [Google Scholar]
- Speisky, H.; Cassels, B.K. Boldo and boldine: An emerging case of natural drug development. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc 1994, 29, 1–12. [Google Scholar]
- Rakotonirainy, M.S.; Lavédrine, B. Screening for antifungal activity of essential oils and related compounds to control the biocontamination in libraries and archives storage areas. Int. Biodeterior. Biodegrad 2005, 55, 141–147. [Google Scholar]
- Del Valle, J.M.; Rogalinski, T.; Zetzl, C.; Brunner, G. Extraction of boldo (Peumus boldus M.) leaves with supercritical CO2 and hot pressurized water. Food Res. Int 2005, 38, 203–213. [Google Scholar]
- Cendres, A.; Chemat, F.; Page, D.; le Bourvellec, C.; Markowski, J.; Zbrzezniak, M.; Renard, C.M.G.C.; Plocharski, W. Comparison between microwave hydrodiffusion and pressing for plum juice extraction. LWT Food Sci. Technol 2012, 49, 229–237. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci 2012, 13, 8615–8627. [Google Scholar]
- Petigny, L.; Périno-Issartier, S.; Wajsman, J.; Chemat, F. Batch and continuous ultrasound assisted extraction of boldo leaves (Peumus boldus Mol.). Int. J. Mol. Sci 2013, 14, 5750–5764. [Google Scholar]
- Benyoussef, E.-H.; Saibi, S. Influence of essential oil composition on water distillation kinetics. Flavour Fragr. J 2013, 28, 300–308. [Google Scholar]
- Conseil de l’Europe, Pharmacopée Européenne; Maisonneuve, S.A. (Ed.) Conseil de l’Europe: Sainte Ruffine, France, 1996.
- Filly, A.; Fernandez, X.; Minuti, M.; Visinoni, F.; Cravotto, G.; Chemat, F. Solvent-free microwave extraction of essential oil from aromatic herbs: From laboratory to pilot and industrial scale. Food Chem 2014, 150, 193–198. [Google Scholar]
- Périno-Issartier, S.; Zill-e-Huma Abert-Vian, M.; Chemat, F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess Technol 2011, 4, 1020–1028. [Google Scholar]
- Nkhili, E.; Tomao, V.; El Hajji, H.; EL Boustani, E.-S.; Chemat, F.; Dangles, O. Microwave-assisted water extraction of green tea polyphenols. Phytochem. Anal 2009, 20, 408–415. [Google Scholar]
- Farhat, A.; Ginies, C.; Romdhane, M.; Chemat, F. Eco-friendly and cleaner process for isolation of essential oil using microwave energy. J. Chromatogr. A 2009, 1216, 5077–5085. [Google Scholar]
- Chemat, F.; Lucchesi, M.E.; Smadja, J.; Favretto, L.; Colnaghi, G.; Visinoni, F. Microwave accelerated steam distillation of essential oil from lavender: A rapid, clean and environmentally friendly approach. Anal. Chim. Acta 2006, 555, 157–160. [Google Scholar]






| Time (min/coded) | Power (W/coded) | NVOC Yield (%) | VOC Yield (%) | ||
|---|---|---|---|---|---|
| 20 | −1 | 200 | −1 | 24 | 0.6 |
| 40 | 0 | 200 | −1 | 24.6 | 1.2 |
| 60 | 1 | 200 | −1 | 25 | 1.4 |
| 20 | −1 | 550 | 0 | 24.9 | 0.6 |
| 40 | 0 | 550 | 0 | 24.1 | 1.2 |
| 60 | 1 | 550 | 0 | 25.9 | 1.4 |
| 20 | −1 | 900 | 1 | 23.1 | 0.6 |
| 40 | 0 | 900 | 1 | 23.3 | 1.4 |
| 60 | 1 | 900 | 1 | 23.2 | 1.4 |
| 40 | 0 | 550 | 0 | 25.1 | 1.2 |
| 40 | 0 | 550 | 0 | 24.7 | 1.2 |
| 40 | 0 | 550 | 0 | 24.2 | 1.2 |
| 40 | 0 | 550 | 0 | 24.7 | 1.2 |
| 40 | 0 | 550 | 0 | 24.9 | 1.2 |
| Results | Lab Scale MAE | Conventional Hydrodistillation | Scale up MAE |
|---|---|---|---|
| Dry mass percentage (%) | 2.11 | 2.83 | 2.10 |
| NVOC yield (%) | 25.67 | 24.90 | 26.1 |
| VOC yield (%) | 1.4 | 1.4 | 1.4 |
| Boldine (ppm) | 122.4 | 105.4 | 129 |
| Total time of extraction (min) | 56 | 146 | 56 |
| Energy consumption (J/g boldo) | 1344 | 5256 | 1344 |
| N° | Compounds a | HD (%) | MAE Scale up (%) | Lab Scale MAE | RI b |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | 35.8 | 47.1 | 40.4 | ||
| 1 | Thujene alpha | 0.1 | 0.1 | 0.2 | 924 |
| 2 | Pinene alpha | 1.0 | 0.9 | 1.2 | 931 |
| 3 | Camphene | 0.2 | 0.2 | 0.2 | 945 |
| 4 | Sabinene | 1.0 | 1.3 | 1.1 | 971 |
| 5 | Pinene beta | 0.4 | 0.5 | 0.5 | 974 |
| 6 | Myrcene beta | 0.2 | - | 0.2 | 990 |
| 7 | Phellandrene alpha | - | - | 0.1 | 1004 |
| 8 | 3-Carene | 0.2 | 0.2 | 0.2 | 1009 |
| 9 | Alpha. Terpinene | 0.3 | 0.4 | 0.4 | 1015 |
| 10 | Para. Cymene | 12.9 | 19.0 | 16.8 | 1026 |
| 11 | d-Limonene | 18.8 | 23.2 | 18.5 | 1033 |
| 12 | Gamma. Terpinene | 0.4 | 0.7 | 0.6 | 1057 |
| 13 | Terpinolene | 0.4 | 0.5 | 0.4 | 1087 |
| Oxygenated monoterpenes | 57.3 | 47.4 | 50.4 | ||
| 14 | Linalol | 1.0 | 1.6 | 0.9 | 1101 |
| 15 | Fenchol | 0.2 | 0.3 | 0.2 | 1111 |
| 16 | Cis, para. 2-Menthen-1-ol | 0.4 | 0.3 | 0.3 | 1118 |
| 17 | Campholenic Aldehyde | - | 0.1 | - | 1122 |
| 18 | Para. 2,8-Menthadien-1-ol, cis | 0.1 | - | 0.1 | 1131 |
| 19 | Trans Pinocarveol | 0.6 | 0.6 | 0.5 | 1133 |
| 20 | Trans-p-2-menthen-1-ol | 0.1 | 0.2 | 0.1 | 1135 |
| 21 | Camphor | 0.2 | 0.2 | 0.1 | 1138 |
| 22 | Sabina ketone | 0.2 | 0.2 | 0.2 | 1152 |
| 23 | Pinocarvone | 0.6 | 0.9 | 0.7 | 1156 |
| 24 | Borneol | - | 0.1 | - | 1160 |
| 25 | 1-Terpinen-4-ol | 2.2 | 2.0 | 1.7 | 1173 |
| 26 | Cryptone | 0.3 | 0.2 | 0.3 | 1181 |
| 27 | Alpha-terpineol | 2.2 | 1.6 | 1.4 | 1188 |
| 28 | Myrtenal | 0.5 | 0.7 | 0.6 | 1192 |
| 29 | Ascaridole | 46.9 | 36.5 | 40.9 | 1242 |
| 30 | Dérivé Ascaridole | 0.3 | 0.5 | - | 1252 |
| 31 | Thymol | 0.3 | 0.3 | 0.4 | 1288 |
| 32 | Carvacrol | 0.2 | 0.2 | 0.3 | 1297 |
| 33 | Methyl Eugenol | 0.9 | 0.8 | 1.1 | 1405 |
| 34 | Spathulenol | 0.4 | 0.3 | 0.7 | 1572 |
| Oxygenated sesquiterpenes | 0.2 | 0.3 | 1.0 | ||
| 35 | Nerolidol (E) | - | - | 0.2 | 1568 |
| 36 | Caryophyllene oxide Isomer 1 | - | 0.1 | 0.2 | 1575 |
| 37 | Viridiflorol | - | - | 0.2 | 1592 |
| 38 | Alpha.-Cadinol | - | - | 0.1 | 1649 |
| 39 | Alpha.-Bisabolol | 0.2 | 0.2 | 0.4 | 1681 |
| Other oxygenated compound | 0.2 | 0.3 | 0.3 | ||
| 40 | Bornyl acetate | 0.2 | 0.3 | 0.3 | 1282 |
| Extraction time (min) | 150.0 | 56.0 | 56.0 | ||
| Yield (%) | 1.4 | 1.4 | 1.4 | ||
| Total oxygenated compounds | 57.8 | 48.0 | 51.7 | ||
| Total non oxygenated compounds | 35.8 | 47.1 | 40.4 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Petigny, L.; Périno, S.; Minuti, M.; Visinoni, F.; Wajsman, J.; Chemat, F. Simultaneous Microwave Extraction and Separation of Volatile and Non-Volatile Organic Compounds of Boldo Leaves. From Lab to Industrial Scale. Int. J. Mol. Sci. 2014, 15, 7183-7198. https://doi.org/10.3390/ijms15057183
Petigny L, Périno S, Minuti M, Visinoni F, Wajsman J, Chemat F. Simultaneous Microwave Extraction and Separation of Volatile and Non-Volatile Organic Compounds of Boldo Leaves. From Lab to Industrial Scale. International Journal of Molecular Sciences. 2014; 15(5):7183-7198. https://doi.org/10.3390/ijms15057183
Chicago/Turabian StylePetigny, Loïc, Sandrine Périno, Matteo Minuti, Francesco Visinoni, Joël Wajsman, and Farid Chemat. 2014. "Simultaneous Microwave Extraction and Separation of Volatile and Non-Volatile Organic Compounds of Boldo Leaves. From Lab to Industrial Scale" International Journal of Molecular Sciences 15, no. 5: 7183-7198. https://doi.org/10.3390/ijms15057183






