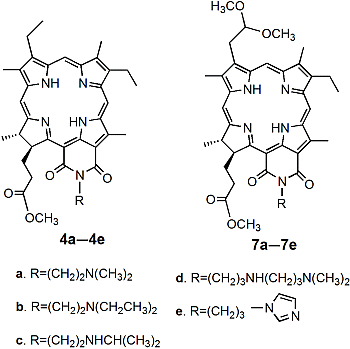Synthesis and Characterization of Novel Purpurinimides as Photosensitizers for Photodynamic Therapy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. In Vitro Study
2.3. Singlet Oxygen Study
2.4. The Hydrophobicity Property Study
3. Experimental Section
3.1. General Methods
3.2. Preparation of Mesopurpurin-18-N-aminoimides
3.2.1. General Procedure
3.2.2. Characteristic Data for Mesopurpurin-18-N-(N,N-dimethyl)ethylimide 4a
3.2.3. Characteristic Data for Mesopurpurin-18-N-(N,N-diethyl)ethylimide 4b
3.2.4. Characteristic Data for Mesopurpurin-18-N-(N-isopropylamino)ethylimide 4c
3.2.5. Characteristic Data for Mesopurpurin-18-N-(N,N-dimethylpropylamino)propylimide 4d
3.2.6. Characteristic Data for Mesopurpurin-18-N-(imidazolyl)propylimide 4e
3.3. Preparation of 3-(2,2-Dimethoxyethyl)-3-devinyl-purpurin-18-N-aminoimides
3.3.1. General Procedure
3.3.2. Characteristic Data for 3-(2,2-Dimethoxyethyl)-3-devinyl-purpurin-18-N-(N,N-dimethyl)ethyl-imide 7a
3.3.3. Characteristic Data for 3-(2,2-Dimethoxyethyl)-3-devinyl-purpurin-18-N-(N,N-diethyl)ethyl-imide 7b
3.3.4. Characteristic Data for 3-(2,2-Dimethoxyethyl)-3-devinyl-purpurin-18-N-(N-isopropylamino)-ethylimide 7c
3.3.5. Characteristic Data for 3-(2,2-Dimethoxyethyl)-3-devinyl-purpurin-18-N-(N,N-dimethylpropyl-amino)propylimide 7d
3.3.6. Characteristic Data for 3-(2,2-Dimethoxyethyl)-3-devinyl-purpurin-18-N-(imidazolyl)-propylimide 7e
3.4. In Vitro Photosensitizing Efficacy
3.5. Measurement of Singlet Oxygen Photogeneration
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsB.C.C. and J.Z.L. designed the study. B.C.C. performed the data collection and data analysis. B.C.C. performed the experiments and drafted the manuscript. B.C.C. and I.Y. made critical revisions to the paper. I.Y., W.K.L. and Y.K.S. obtained funding and supervised the study. All authors discussed the results and commented on the manuscript.
References
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol 1992, 55, 145–157. [Google Scholar]
- Macdonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar]
- Dougherty, T.J.; Gomer, C.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst 1998, 90, 889–905. [Google Scholar]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of singlet oxygen as the cytotoxic agent in photo-inactivation of a murine tumor. Cancer Res 1976, 36, 2326–2329. [Google Scholar]
- Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat 2005, 4, 283–293. [Google Scholar]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol 2004, 5, 497–508. [Google Scholar]
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem 2004, 47, 3897–3915. [Google Scholar]
- Sternberg, E.D.; Dolphin, D.; Brückner, C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar]
- Zheng, G.; Potter, W.R.; Camacho, S.H.; Missert, J.R.; Wang, G.; Bellnier, D.A.; Henderson, B.W.; Rodgers, M.A.J.; Dougherty, T.J.; Pandey, R.K. Synthesis, photophysical properties, tumor uptake, and preliminary in vivo photosensitizing efficacy of a homologous series of 3-(1′-alkyloxy)ethyl-3-devinylpurpurin-18-N-alkylimides with variable lipophilicity. J. Med. Chem 2001, 44, 1540–1559. [Google Scholar]
- Yoon, I.; Park, H.S.; Cui, B.C.; Shim, Y.K. Synthesis and photodynamic activities of pyrazolyl and cyclopropyl derivatives of purpurin-18 methyl ester and purpurin-18-N-butylimide. Bull. Korean Chem. Soc 2011, 32, 169–174. [Google Scholar]
- Li, J.Z.; Li, L.; Kim, J.H.; Cui, B.C.; Shim, Y.K.; Wang, J.J. Efficient synthesis and in vitro photodynamic anticancer study of new purpurinimide-hydrazone conjugates. J. Porphyr. Phthalocyanines 2011, 15, 264–270. [Google Scholar]
- Li, J.Z.; Wang, J.J.; Yoon, I.; Cui, B.C.; Shim, Y.K. Synthesis of novel long wavelength cationic chlorins via stereoselective aldol-like condensation. Bioorg. Med. Chem. Lett 2012, 22, 1846–1849. [Google Scholar]
- Cui, B.C.; Cha, M.U.; Li, J.Z.; Park, H.S.; Yoon, I.; Shim, Y.K. Efficient synthesis and in vitro PDT effect of purpurin-18-N-aminoimides. Bull. Korean Chem. Soc 2010, 31, 3313–3317. [Google Scholar]
- Henderson, B.W.; Bellinier, D.A.; Graco, W.R.; Sharma, A.; Pandey, R.K.; Vaughan, L.; Weishaupt, K.R.; Dougherty, T.J. A quantitative structure–activity relationship for a congeneric series of pyropheophorbide derivatives as photosensitizers for photodynamic therapy. Cancer Res 1997, 57, 4000–4007. [Google Scholar]
- Wang, K.; Poon, C.T.; Choi, C.Y.; Wong, W.-K.; Kwong, D.W.J.; Yu, F.Q.; Zhang, H.; Li, Z.Y. Synthesis, circular dichroism, DNA cleavage and singlet oxygen photogeneration of 4-amidinophenyl porphyrins. J. Porphyr. Phthalocyanines 2012, 16, 85–92. [Google Scholar]
- Smith, K.M.; Goff, D.A.; Simpson, D.J. The meso substitution of chlorophyll derivatives: Direct route for transformation of bacteriopheophorbides d into bacteriopheophorbides c. J. Am. Chem. Soc 1985, 107, 4946–4954. [Google Scholar]
- Han, G.F.; Wang, J.J.; Qu, Y.; Shim, Y.K. Synthesis of purpurin-18 imide derivatives. Chin. J. Org. Chem 2005, 25, 319–326. [Google Scholar]






| Compound | Absorption λmax (nm) (log ɛ) a | |||
|---|---|---|---|---|
| Soret | ΔSoret (Δɛ) | Qy | ΔQy (Δɛ) | |
| 3 | 410.8 (0.91) | 0 | 686.7 (0.31) | 0 |
| 4a | 417.3 (0.92) | 6.5 (0.01) | 695.8 (0.29) | 9.1 (−0.02) |
| 4b | 417.4 (0.93) | 6.6 (0.02) | 696.4 (0.28) | 9.7 (−0.03) |
| 4c | 418.3 (0.91) | 7.5 (0) | 697.5 (0.27) | 10.8 (−0.04) |
| 4d | 417.3 (0.91) | 6.5 (0) | 694.9 (0.26) | 8.2 (−0.05) |
| 4e | 417.4 (0.92) | 6.6 (0.01) | 695.2 (0.25) | 8.5 (−0.06) |
| 6 | 411.5 (1.16) | 0 | 690.3 (0.33) | 0 |
| 7a | 417.4 (1.19) | 5.9 (0.03) | 700.1 (0.36) | 9.8 (0.03) |
| 7b | 417.2 (1.19) | 5.7 (0.03) | 699.6 (0.31) | 9.3 (−0.02) |
| 7c | 418.1 (1.21) | 6.6 (0.05) | 703.6 (0.35) | 13.3 (0.02) |
| 7d | 417.5 (1.21) | 6.0 (0.05) | 698.9 (0.35) | 8.6 (0.02) |
| 7e | 417.4 (1.17) | 5.9 (0.01) | 698.8 (0.37) | 8.5 (0.04) |
| Compound | 4a | 4b | 4c | 7a | 7b | 7c |
|---|---|---|---|---|---|---|
| IC50 a (μM) | 0.28 | 0.37 | 0.40 | 0.95 | 1.27 | 0.70 |
| Compound | Log P |
|---|---|
| 4a | 6.94 ± 1.62 |
| 4b | 8.00 ± 1.62 |
| 4c | 7.32 ± 1.62 |
| 4d | 6.89 ± 1.63 |
| 4e | 6.88 ± 1.62 |
| 7a | 5.80 ± 1.64 |
| 7b | 6.87 ± 1.64 |
| 7c | 6.19 ± 1.64 |
| 7d | 5.76 ± 1.65 |
| 7e | 5.75 ± 1.64 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cui, B.C.; Yoon, I.; Li, J.Z.; Lee, W.K.; Shim, Y.K. Synthesis and Characterization of Novel Purpurinimides as Photosensitizers for Photodynamic Therapy. Int. J. Mol. Sci. 2014, 15, 8091-8105. https://doi.org/10.3390/ijms15058091
Cui BC, Yoon I, Li JZ, Lee WK, Shim YK. Synthesis and Characterization of Novel Purpurinimides as Photosensitizers for Photodynamic Therapy. International Journal of Molecular Sciences. 2014; 15(5):8091-8105. https://doi.org/10.3390/ijms15058091
Chicago/Turabian StyleCui, Bing Cun, Il Yoon, Jia Zhu Li, Woo Kyoung Lee, and Young Key Shim. 2014. "Synthesis and Characterization of Novel Purpurinimides as Photosensitizers for Photodynamic Therapy" International Journal of Molecular Sciences 15, no. 5: 8091-8105. https://doi.org/10.3390/ijms15058091
APA StyleCui, B. C., Yoon, I., Li, J. Z., Lee, W. K., & Shim, Y. K. (2014). Synthesis and Characterization of Novel Purpurinimides as Photosensitizers for Photodynamic Therapy. International Journal of Molecular Sciences, 15(5), 8091-8105. https://doi.org/10.3390/ijms15058091