Metabolic Profiling of Somatic Tissues from Monochamus alternatus (Coleoptera: Cerambycidae) Reveals Effects of Irradiation on Metabolism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Metabolic Profiling of Untreated and Irradiated Samples by GC-TOF/MS
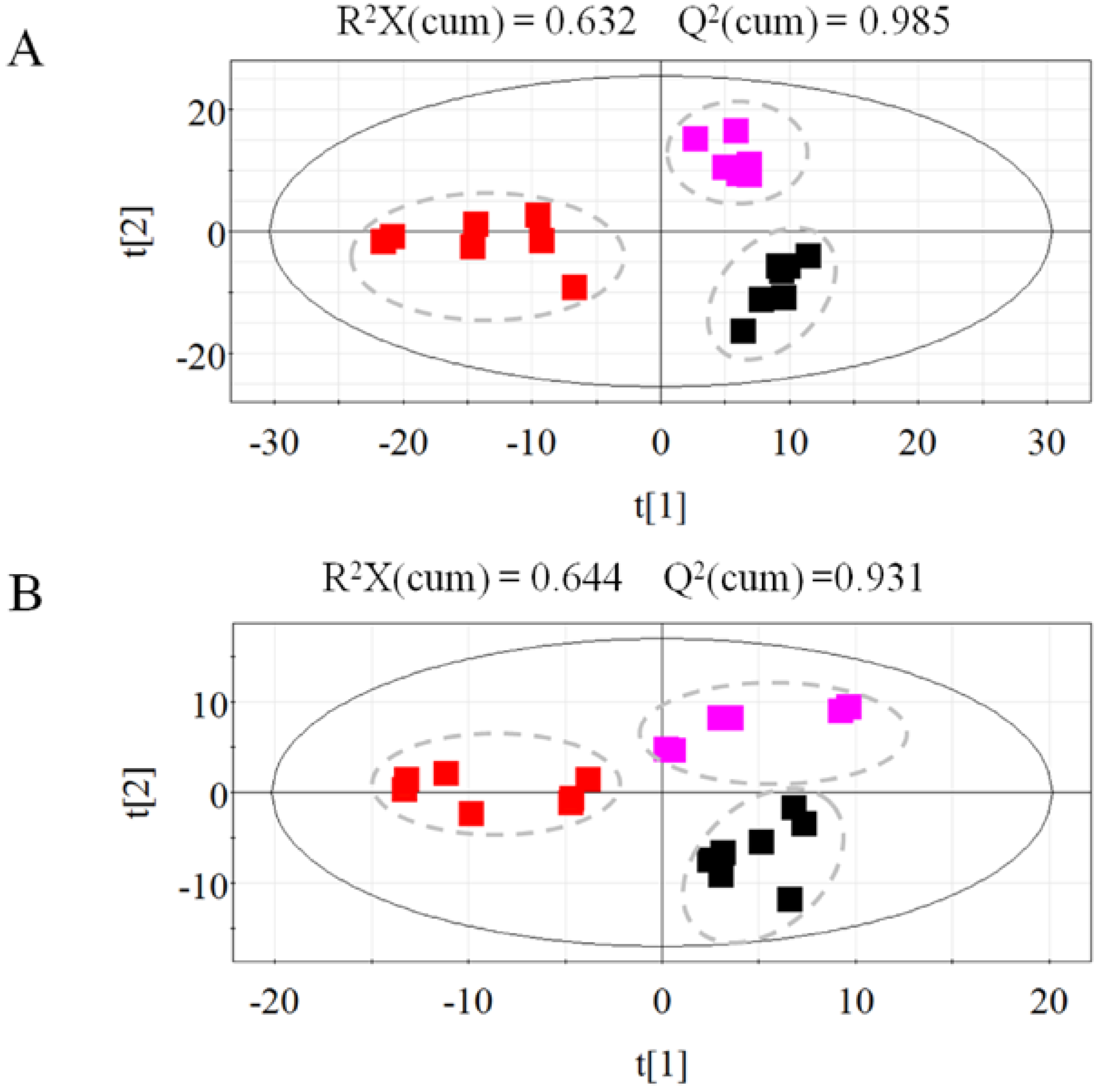
2.1.2. Metabolic Profiling of Untreated and Irradiated Samples by GC-TOF/MS

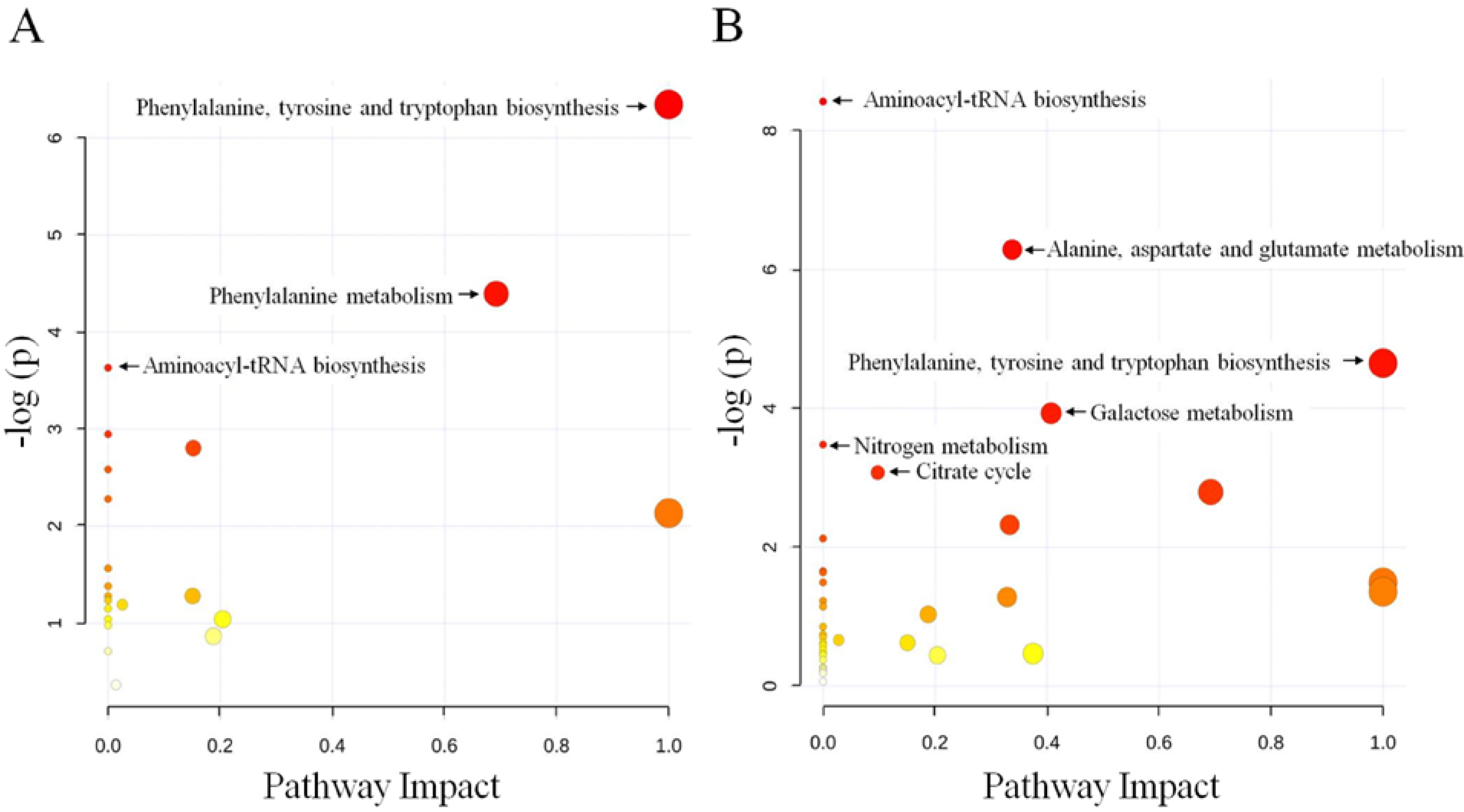
2.2. Discussion
3. Experimental Section
3.1. Insects and Irradiation
3.2. Chemicals
3.3. Metabolite Extraction and Derivatization
3.4. GC-TOF/MS
3.5. Data Processing
3.6. Univariate and Multivariate Statistical Analyses
3.7. Hierarchical Clustering and Pathway Enrichment Analysis
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Robinson, A.S.; Cayol, J.P.; Hendrichs, J. Recent findings on medfly sexual behavior: Implications for SIT. FL Entomol. 2002, 85, 171–181. [Google Scholar] [CrossRef]
- Sakurai, H.; Watanabe, H.; Sugimoto, T.; Sakuratani, Y.; Setoguchi, O.; Kawazoe, K. Effects of gamma-radiation on the gametogenesis in the sweetpotato [Ipomoea batatas] weevil, Cylas formicarius. Res. Bull. Fac. Agric. Gifu Univ. 1994, 59, 11–20. [Google Scholar]
- Sakurai, H.; Murakami, Y.; Kohama, T.; Teruya, T. Sterilizing mechanism of gamma-radiation in the female of West Indian sweet potato weevil, Euscepes postfasciatus. Res. Bull. Fac. Agri. Gifu Univ. 2000, 65, 13–20. [Google Scholar]
- Sakurai, H.; Murakami, Y.; Utimura, H.; Kohama, T.; Teruya, T. Sterilizing mechanism of gamma-radiation in the male of West Indian Sweet Potato weevil, Euscepes postfasciatus. Res. Bull. Fac. Agric. Gifu Univ. 2000, 65, 5–12. [Google Scholar]
- Parker, A.; Mehta, K. Sterile insect technique: A model for dose optimization for improved sterile insect quality. FL Entomol. 2007, 90, 88–95. [Google Scholar] [CrossRef]
- Toledo, J.; Rull, J.; Oropeza, A.; Hernández, E.; Liedo, P. Irradiation of Anastrepha obliqua (Diptera: Tephritidae) revisited: Optimizing sterility induction. J. Econ. Entomol. 2004, 97, 383–389. [Google Scholar] [CrossRef]
- Rull, J.; Diaz-Fleischer, F.; Arredondo, J. Irradiation of Anastrepha ludens (Diptera: Tephritidae) revisited: Optimizing sterility induction. J. Econ. Entomol. 2007, 100, 1153–1159. [Google Scholar] [CrossRef]
- Kumano, N.; Kuriwada, T.; Shiromoto, K.; Haraguchi, D.; Kohama, T. Assessment of effect of partial sterility on mating performance in sweetpotato weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 2010, 103, 2034–2041. [Google Scholar] [CrossRef]
- Kumano, N.; Kuriwada, T.; Shiromoto, K.; Haraguchi, D.; Kohama, T. Evaluation of partial sterility in mating performance and reproduction of the West Indian sweetpotato weevil, Euscepes postfasciatus. Entomol. Exp. Appl. 2010, 136, 45–52. [Google Scholar] [CrossRef]
- Kumano, N.; Kuriwada, T.; Shiromoto, K.; Haraguchi, D.; Kohama, T. Fractionated irradiation improves the mating performance of the West Indian sweet potato weevil Euscepes postfasciatus. Agric. For. Entomol. 2011, 13, 349–356. [Google Scholar] [CrossRef]
- Akbulut, S.; Stamps, W.T. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2012, 42, 89–99. [Google Scholar] [CrossRef]
- Fan, J.T.; Sun, J.H. Influences of host volatiles on feeding behaviour of the Japanese pine sawyer, Monochamus alternatus. J. Appl. Entomol. 2006, 130, 238–244. [Google Scholar] [CrossRef]
- Song, L.; Liu, X.X.; Zhang, Y.A.; Zhang, Q.W.; Zhao, Z.W. The cloning and expression of α-tubulin in Monochamus alternatus. Insect Mol. Biol. 2008, 17, 495–504. [Google Scholar] [CrossRef]
- Mamiya, Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef]
- Togashi, K. Population density of Monochamus alternatus adults (Coleoptera: Cerambycidae) and incidence of pine wilt disease caused by Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae). Res. Popul. Ecol. 1988, 30, 177–192. [Google Scholar] [CrossRef]
- Mou, J.J.; Zhang, Y.A.; Li, M.L.; Jiang, P.; Wang, Y.Z. Research on the sterile effect of radiation on Monochamus alternatus. Sci. Silvae Sin. 2005, 41, 207–210. (In Chinese) [Google Scholar]
- Ma, P.P.; Zhang, Y.A.; Wang, D.Y.; Wang, Y.Z.; Qu, L.J.; Jiang, P.; Mou, J.J. Influence of 60Coγ irradiation on fertility of Japanese pine sawyer beetle Monochamus alternatus (Coleoptera: Cerambycidae). J. Nucl. Agric. Sci. 2008, 22, 101–104. (In Chinese) [Google Scholar]
- Al-Rubeai, M.; Fussenegger, M. Systems Biology; Springe: Heidelberg, The Netherlands, 2007. [Google Scholar]
- Griffin, J.L.; Bollard, M.E. Metabonomics: Its potential as a tool in toxicology for safety assessment and data integration. Curr. Drug Metab. 2004, 5, 389–398. [Google Scholar] [CrossRef]
- Bino, R.J.; Hall, R.D.; Fiehn, O.; Kopka, J.; Saito, K.; Draper, J.; Nikolau, B.J.; Mendes, P.; Roessner-Tunali, U.; Beale, M.H.; et al. Potential of metabolomics as a functional genomics tool. Trends Plant Sci. 2004, 9, 418–425. [Google Scholar] [CrossRef]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.D.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; Ollas, C.D.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, B.; Su, M.; Baxter, S.; Zheng, X.; Zhao, X.; Yen, Y.; Jia, W. Mass spectrometry-based quantitative metabolomics revealed a distinct lipid profile in breast cancer patients. Int. J. Mol. Sci. 2013, 14, 8047–8061. [Google Scholar]
- Teixeira, A.; Martins, V.; Noronha, H.; Eiras-Dias, J.; Gerós, H. The first insight into the metabolite profiling of grapes from three Vitis vinifera L. cultivars of two controlled appellation (DOC) regions. Int. J. Mol. Sci. 2014, 15, 4237–4254. [Google Scholar] [CrossRef]
- Tyburski, J.B.; Patterson, A.D.; Krausz, K.W.; Slavik, J.; Fornace, A.J.; Gonzalez, F.J.; Idle, J.R. Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiat. Res. 2008, 170, 1–14. [Google Scholar] [CrossRef]
- Rocha, C.M.; Barros, A.S.; Gil, A.M.; Goodfellow, B.J.; Humpfer, E.; Spraul, M.; Carreira, I.M.; Melo, J.B.; Bernardo, J.; Gomes, A.; et al. Metabolic profiling of human lung cancer tissue by 1H high resolution magic angle spinning (HRMAS) NMR spectroscopy. J. Proteome Res. 2009, 9, 319–332. [Google Scholar]
- Calvani, R.; Miccheli, A.; Capuani, G.; Miccheli, A.T.; Puccetti, C.; Delfini, M.; Iaconelli, A.; Nanni, G.; Mingrone, G. Gut microbiome-derived metabolites characterize a peculiar obese urinary metabotype. Int. J. Obes. 2010, 34, 1095–1098. [Google Scholar] [CrossRef]
- Johnson, C.H.; Patterson, A.D.; Krausz, K.W.; Lanz, C.; Kang, D.W.; Luecke, H.; Gonzalez, F.J.; Idle, J.R. Radiation metabolomics. 4. UPLC-ESI-QTOFMS-based metabolomics for urinary biomarker discovery in gamma-irradiated rats. Radiat. Res. 2011, 175, 473–484. [Google Scholar] [CrossRef]
- Cheema, A.K.; Suman, S.; Kaur, P.; Singh, R.; Fornace, A.J., Jr.; Datta, K. Long-term differential changes in mouse intestinal metabolomics after γ and heavy ion radiation exposure. PLoS One 2014, 9, e87079. [Google Scholar]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef]
- Shipley, M.M.; Wellington, K.; Rao, A.; Ritchie, T.; Vogtsberger, R.C. Fatty acid composition of a burrowing mayfly, Hexagenia limbata (Ephemeroptera: Ephemeridae), from a North Central Texas Lake. J. Kansas Entomol. Soc. 2012, 85, 245–258. [Google Scholar] [CrossRef]
- Zaghloul, Y.S.; Abbassy, S.A.; Elakhdar, E.A.H. The effect of e-Selen antioxidant on the fatty acids content of the homogenate of unirradiated and irradiated pupae of Ceratitis Capitata. J. Radiat. Res. Appl. Sci. 2011, 4, 289–299. [Google Scholar]
- Aitken, R.J.; Harkeness, D.; Buckingham, D.W. Analysis of lipid peroxidation mechanism in human sperm. Mol. Reprod. Dev. 2006, 35, 302–315. [Google Scholar]
- Wang, Y.; Lin, D.S.; Bolewicz, L.; Connor, W.E. The predominance of polyunsaturated fatty acids in the butterfly Morpho peleides before and after metamorphosis. J. Lipid Res. 2006, 47, 530–536. [Google Scholar]
- Lauzon, C.R.; Potter, S.E. Description of the irradiated and nonirradiated midgut of Ceratitis capitata Wiedemann (Diptera: Tephritidae) and Anastrepha ludens Loew (Diptera: Tephritidae) used for sterile insect technique. J. Pest Sci. 2012, 85, 217–226. [Google Scholar] [CrossRef]
- Eberhart, C.E.; Dubois, R.N. Eicosanoids and the gastrointestinal tract. Gastroenterology 1995, 109, 285–301. [Google Scholar] [CrossRef]
- Chen, P.S. Amino acid and protein metabolism. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: New York, NY, USA, 1985; Volume 10, pp. 177–219. [Google Scholar]
- Kim, P.M.; Duan, X.; Huang, A.S.; Liu, C.Y.; Ming, G.L.; Song, H.; Snyder, S.H. Aspartate racemase, generating neuronal d-aspartate, regulates adult neurogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3175–3179. [Google Scholar]
- Horio, M.; Ishima, T.; Fujita, Y.; Inoue, R.; Mori, H.; Hashimoto, K. Decreased levels of free d-aspartic acid in the forebrain of serine racemase (Srr) knock-out mice. Neurochem. Int. 2013, 62, 843–847. [Google Scholar] [CrossRef]
- Lee, D.Y.; Bowen, B.P.; Nguyen, D.H.; Parsa, S.; Huang, Y.; Mao, J.H.; Northen, T.R. Low-dose ionizing radiation-induced blood plasma metabolic response in a diverse genetic mouse population. Radiat. Res. 2012, 178, 551–555. [Google Scholar] [CrossRef]
- Boctor, I.Z. Some effects of radiation on the free amino acids of adult female Mediterranean fruit fly, Ceratitis capitata Wied. Experientia 1980, 36, 36–37. [Google Scholar] [CrossRef]
- Burns, G.A.; Stephens, K.E. Expression of mRNA for the N-methyl-d-aspartate (NMDAR1) receptor and vasoactive intestinal polypeptide (VIP) co-exist in enteric neurons of the rat. J. Auton. Nerv. Syst. 1995, 55, 207–210. [Google Scholar] [CrossRef]
- Fukunaga, Y.; Kimura, M.; Saitoh, O. NMDA receptor in intestinal enteroendocrine cell, STC-1. Neuroreport 2010, 21, 772–776. [Google Scholar] [CrossRef]
- Chippendale, G.M. The functions of carbohydrates in insect life processes. In Biochemistry of Insects; Rockstein, M., Ed.; Academic Press: New York, NY, USA, 1978; pp. 1–55. [Google Scholar]
- Gibbs, A.; Pomonis, J.G. Physical properties of insect cuticular hydrocarbons: The effects of chain length, methyl-branching and unsaturation. Comp. Biochem. Phys. B 1995, 112, 243–249. [Google Scholar] [CrossRef]
- Gibbs, A.G. Water-proofing properties of cuticular lipids. Am. Zool. 1998, 38, 471–482. [Google Scholar]
- Blomquist, G.J.; Bagnères, A.G. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Cambridge University Press: Cambridge, UK, 2010; p. 492. [Google Scholar]
- Fukaya, M.; Akino, T.; Yasuda, T.; Wakamura, S.; Satoda, S.; Senda, S. Hydrocarbon components in contact sex pheromone of the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae) and pheromonal activity of synthetic hydrocarbons. Entomol. Sci. 2000, 3, 211–218. [Google Scholar]
- Christoph Brändli, J.M.; Vereecken, N.J.; Schulz, C.M.; Francke, W.; Schiestl, F.P. Cuticular hydrocarbons as sex pheromone of the bee Colletes cunicularius and the key to its mimicry by the sexually deceptive orchid, Ophrys exaltata. J. Chem. Ecol. 2005, 31, 1765–1787. [Google Scholar] [CrossRef]
- Herzner, G.; Schmitt, T.; Heckel, F.; Schreier, P.; Strohm, E. Brothers smell similar: Variation in the sex pheromone of male European Beewolves Philanthus triangulum F. (Hymenoptera: Crabronidae) and its implications for inbreeding avoidance. Biol. J. Linn. Soc. 2006, 89, 433–442. [Google Scholar] [CrossRef]
- Monnin, T. Chemical recognition of reproductive status in social insects. Ann. Zool. Fennici 2006, 43, 515–530. [Google Scholar]
- MultiExperiment Viewer. Available online: http://www.tm4.org/mev.html (accessed on 4 April 2013).
- Xia, J.; Mandal, R.; Sinelnikov, I.; Broadhurst, D.; Wishart, D.S. MetaboAnalyst 2.0—A Comprehensive Server for Metabolomic Data Analysis. Available online: http://www.metaboanalyst.ca/Metabo-Analysts (accessed on 2 May 2012).
- Weckwerth, W.; Wenzel, K.; Fiehn, O. Process for the integrated extraction, identification and quantification of metabolites, proteins and RNA to reveal their co-regulation in biochemical networks. Proteomics 2004, 4, 78–83. [Google Scholar] [CrossRef]
- Jonsson, P.; Gullberg, J.; Nordstrom, A.; Kusano, M.; Kowalczyk, M.; Sjöström, M.; Moritz, T. A strategy for identifying differences in large series of metabolomic samples analyzed by GC/MS. Anal. Chem. 2004, 76, 1738–1745. [Google Scholar] [CrossRef]
- Jonsson, P.; Johansson, A.I.; Gullberg, J.; Trygg, J.; Ytygg, J.A.J.; Grung, B.; Marklund, S.; Sjöström, M.; Antti, H.; Moritz, T. High-throughput data analysis for detecting and identifying differences between samples in GC/MS-based metabolomic analyses. Anal. Chem. 2005, 77, 5635–5642. [Google Scholar] [CrossRef]
- Ni, Y.; Su, M.; Lin, J.; Wang, X.; Qiu, Y.; Zhao, A.; Chen, T.L.; Jia, W. Metabolic profiling reveals disorder of amino acid metabolism in four brain regions from a rat model of chronic unpredictable mild stress. FEBS Lett. 2008, 582, 2627–2636. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qu, L.; Wang, L.; Wang, Q.; Wang, Y.; Zhang, Y. Metabolic Profiling of Somatic Tissues from Monochamus alternatus (Coleoptera: Cerambycidae) Reveals Effects of Irradiation on Metabolism. Int. J. Mol. Sci. 2014, 15, 10806-10820. https://doi.org/10.3390/ijms150610806
Qu L, Wang L, Wang Q, Wang Y, Zhang Y. Metabolic Profiling of Somatic Tissues from Monochamus alternatus (Coleoptera: Cerambycidae) Reveals Effects of Irradiation on Metabolism. International Journal of Molecular Sciences. 2014; 15(6):10806-10820. https://doi.org/10.3390/ijms150610806
Chicago/Turabian StyleQu, Liangjian, Lijuan Wang, Qinghua Wang, Yuzhu Wang, and Yongan Zhang. 2014. "Metabolic Profiling of Somatic Tissues from Monochamus alternatus (Coleoptera: Cerambycidae) Reveals Effects of Irradiation on Metabolism" International Journal of Molecular Sciences 15, no. 6: 10806-10820. https://doi.org/10.3390/ijms150610806
APA StyleQu, L., Wang, L., Wang, Q., Wang, Y., & Zhang, Y. (2014). Metabolic Profiling of Somatic Tissues from Monochamus alternatus (Coleoptera: Cerambycidae) Reveals Effects of Irradiation on Metabolism. International Journal of Molecular Sciences, 15(6), 10806-10820. https://doi.org/10.3390/ijms150610806




