RETRACTED: Cellulose Nanocrystals/ZnO as a Bifunctional Reinforcing Nanocomposite for Poly(vinyl alcohol)/Chitosan Blend Films: Fabrication, Characterization and Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of CNCs/ZnO
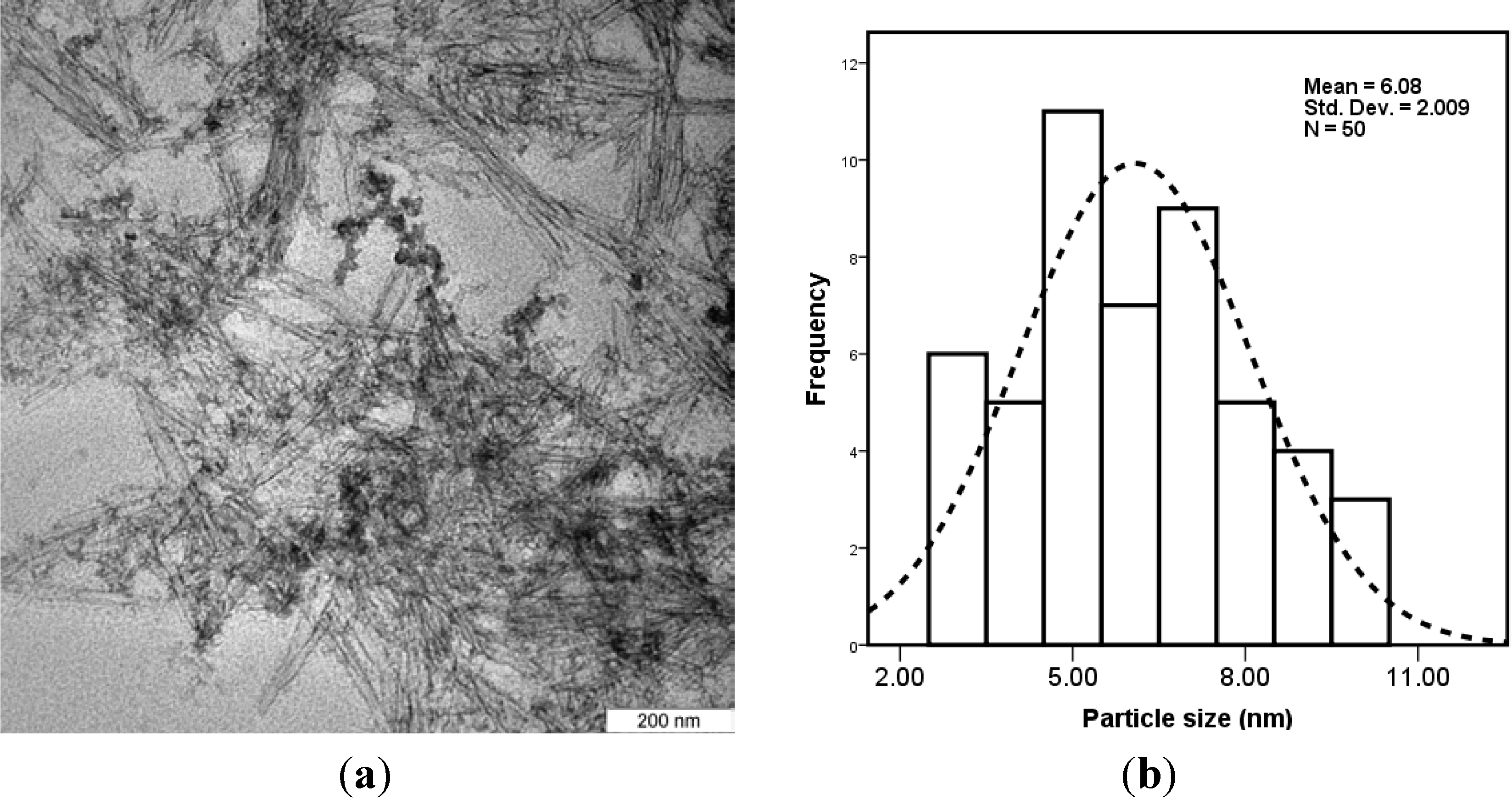
2.2. Characterization of PVA/Cs-Based Films
2.2.1. X-ray Diffraction (XRD)
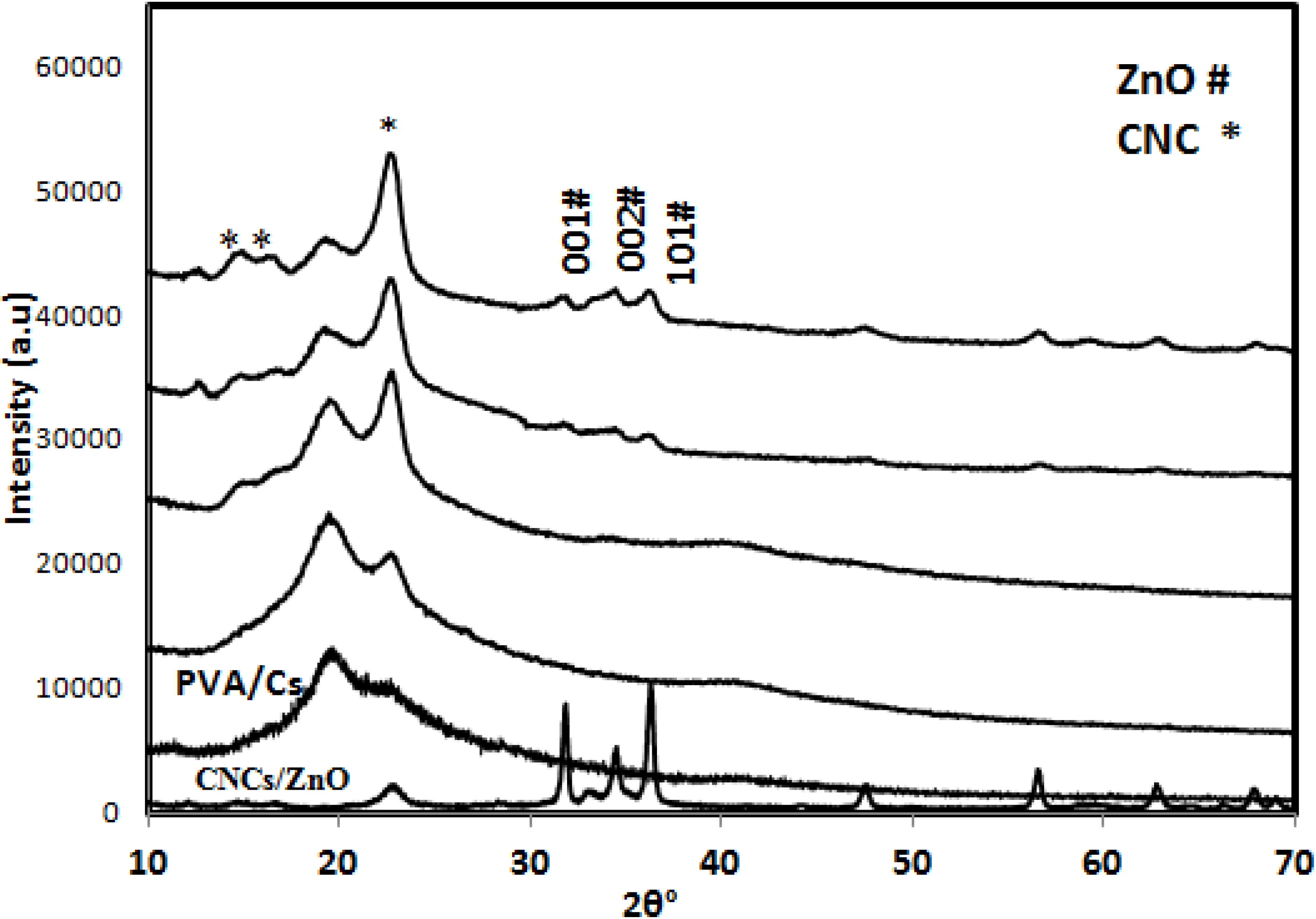
2.2.2. Surface Morphology

2.2.3. Mechanical Properties
| Sample | CNCs/ZnO (wt %) | Ts (MPa) | Tm (MPa) | Eb (mm) |
|---|---|---|---|---|
| PVA/Cs | 0 | 55 ± 1.6 | 395 ± 23 | 10.2 ± 0.2 |
| PVA/Cs/CNCs/ZnO | 1 | 86 ± 1.3 | 691 ± 40 | 6.3 ± 0.9 |
| PVA/Cs/CNCs/ZnO | 3 | 120 ± 2.1 | 825 ± 38 | 5.5 ± 0.4 |
| PVA/Cs/CNCs/ZnO | 5 | 153 ± 1.8 | 932 ± 36 | 3.2 ± 0.7 |
| PVA/Cs/CNCs/ZnO | 7 | 112 ± 2.7 | 801 ± 30 | 4.8 ± 0.3 |
2.2.4. Thermogravimetric Analysis

| Sample | CNCs/ZnO (wt %) | First Step | |
|---|---|---|---|
| Tonset (°C) | T0.5 (°C) | ||
| PVA/Cs | 0 | 217 | 294 |
| PVA/Cs/CNCs/ZnO | 1 | 215 | 328 |
| PVA/Cs/CNCs/ZnO | 3 | 212 | 281 |
| PVA/Cs/ CNCs/ZnO | 5 | 211 | 279 |
| PVA/Cs/CNCs/ZnO | 7 | 209 | 275 |
2.2.5. Ultraviolet Absorbance
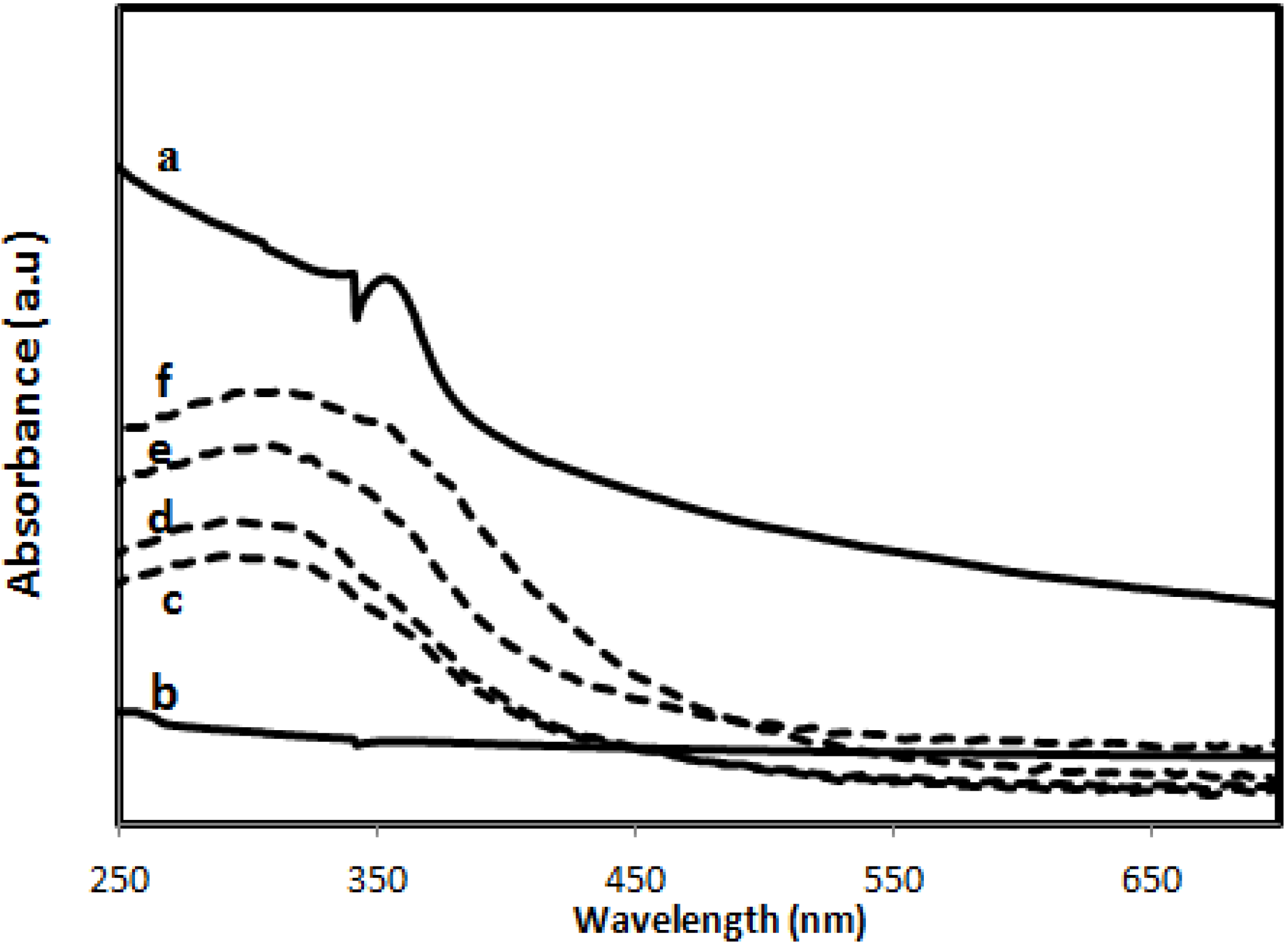
2.2.6. Zinc Release from PVA/Cs/CNCs/ZnO Bio-Nanocomposite
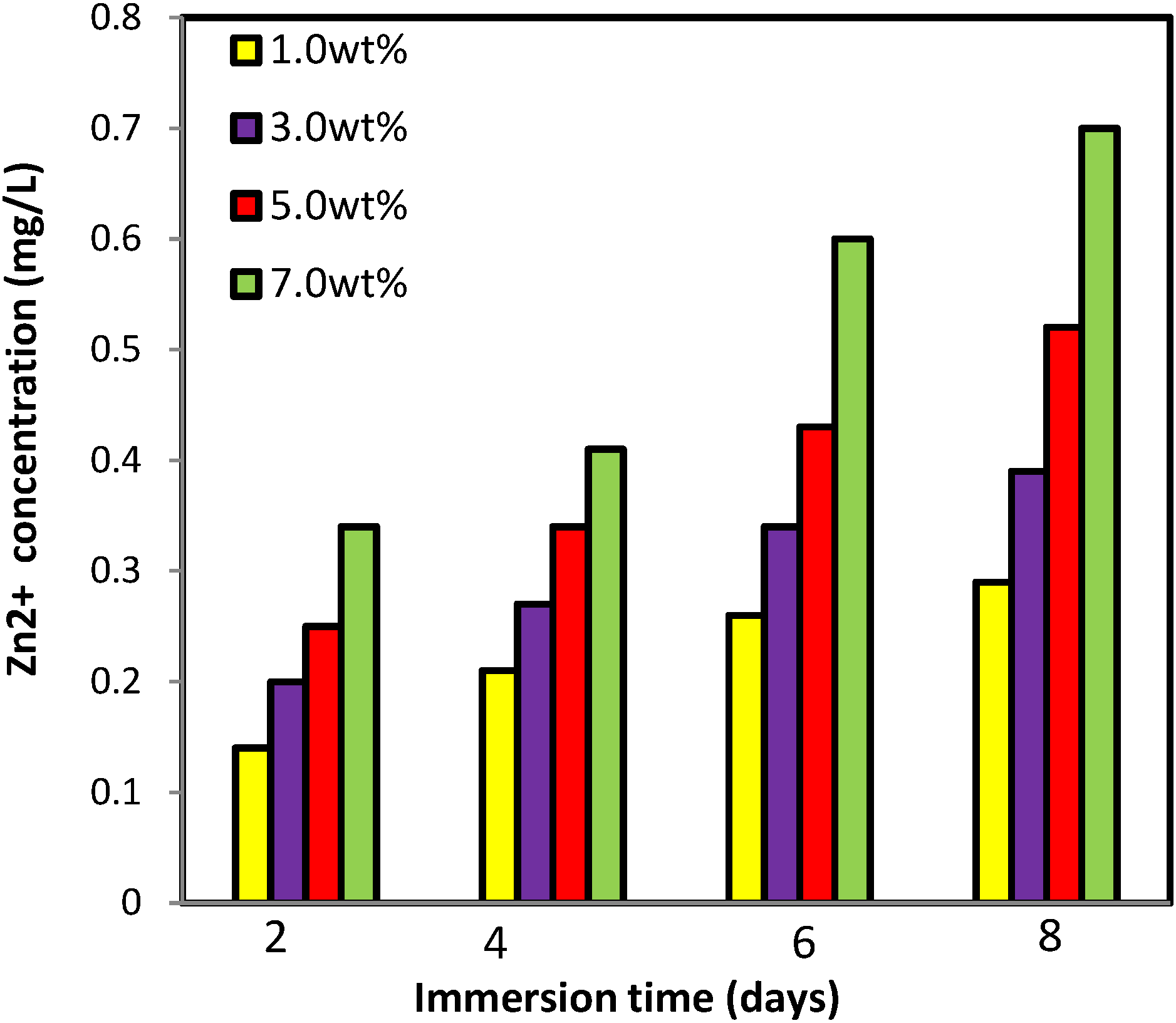
2.2.7. Antibacterial Assessment

| Filler Content wt % | Diameter of Zone (mm) | |
|---|---|---|
| Gram-Positive | Gram-Negative | |
| 0 | - | - |
| 1.0 | - | - |
| 3.0 | 5.4 | - |
| 5.0 | 6.3 | 4.9 |
| 7.0 | 7.1 | 5.4 |
3. Experimental Section
3.1. Materials
3.2. Preparation of CNCs/ZnO Bifunctional Nano-Sized Filler
3.3. Preparation of PVA/Cs/CNCs/ZnO Biocomposite Films
3.4. Characterization
3.5. Zinc Release
3.6. Antibacterial Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, X.; Habibi, Y.; Lucia, L.A. One-pot polymerization, surface grafting, and processing of waterborne polyurethane—Cellulose nanocrystal nanocomposites. J. Mater. Chem. 2009, 19, 7137–7145. [Google Scholar] [CrossRef]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review article: Polymer–matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, selfassembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Liu, H.; Song, J.; Shang, S.; Song, Z.; Wang, D. Cellulose nanocrystal/silver nanoparticle composites as bifunctional nanofillers within waterborne polyurethane. ACS Appl. Mater. Interfaces 2012, 4, 2413–2419. [Google Scholar]
- Kannusamy, P.; Sivalingam, T. ChitosaneZnO/polyaniline hybrid composites: Polymerization of aniline with chitosaneZnO for better thermal and electrical property. Polym. Degrad. Stab. 2013, 98, 988–996. [Google Scholar] [CrossRef]
- Zapata, P.A.; Tamayo, L.; Páez, M.; Cerda, E.; Azócar, I.; Rabagliati, F.M. Nanocomposites based on polyethylene and nanosilver particles produced by metallocenic ‘‘in situ’’ polymerization: Synthesis, characterization, and antimicrobial behavior. Eur. Polym. J. 2011, 47, 1541–1549. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, K.; Lee, Y.; Seo, J.; Seo, K.; Han, H.; Khan, S. Preparation and characterization of UV-cured polyurethane acrylate/ZnO nanocomposite films based on surface modified ZnO. Prog. Org. Coat. 2012, 74, 435–442. [Google Scholar] [CrossRef]
- Li, J.H.; Hong, R.Y.; Li, M.Y.; Li, H.Z.; Zheng, Y.; Ding, J. Effects of ZnO nanoparticles on the mechanical and antibacterial properties of polyurethane coatings. Prog. Org. Coat. 2009, 64, 504–509. [Google Scholar] [CrossRef]
- Seo, J.; Jeon, G.; Jang, E.S.; Bahadar Khan, S.; Han, H. Preparation and properties of poly(propylene carbonate) and nanosized ZnO composite films for packaging applications. J. Appl. Polym. Sci. 2011, 122, 1101–1108. [Google Scholar] [CrossRef]
- Hong, R.; Pan, T.; Qian, J.; Li, H. Synthesis and surface modification of ZnO nanoparticles. Chem. Eng. J. 2006, 119, 71–81. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Zhang, C.-L.; Li, P. Characterisation and cooperative antimicrobial properties of chitosan/nano-ZnO composite nanofibrous membranes. Food Chem. 2012, 132, 419–427. [Google Scholar] [CrossRef]
- Gopal, J.; Wu, H.; Lee, Y. Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry as a rapid and reliable technique for directly evaluating bactericidal activity: Probing the critical concentration of zno nanoparticles as affinity probes. Anal. Chem. 2010, 82, 9617–9621. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S. Nanoporous cellulose as metal nanoparticles support. Biomacromolecules 2009, 10, 87–94. [Google Scholar] [CrossRef]
- Shin, Y.; Bae, I.; Arey, B.W.; Exarhos, G.J. Facile stabilization of gold-silver alloy nanoparticles on cellulose nanocrystal. J. Phys. Chem. 2008, 112, 4844–4848. [Google Scholar] [CrossRef]
- Shin, Y.; Bae, I.T.; Arey, B.W.; Exarhos, G.J. Simple preparation and stabilization of nickel nanocrystals on cellulose nanocrystal. Mater. Lett. 2007, 61, 3215–3217. [Google Scholar] [CrossRef]
- Shin, Y.; Exarhos, G.J. Template synthesis of porous titania using cellulose nanocrystals. Mater. Lett. 2007, 61, 2594–2597. [Google Scholar] [CrossRef]
- Khoo, C.G.L.; Frantzich, S.; Rosinski, A.; Sjostrom, M.; Hoogstraate, J. Permeability studies in chitosan membranes. Effects of crosslinking and poly(ethylene oxide) addition. Eur. J. Pharm. Biopharm. 2003, 55, 47–56. [Google Scholar]
- Wang, Q.; Du, Y.M.; Fan, L.H. Properties of chitosan/poly(vinyl alcohol) films for drug-controlled release. J. Appl. Polym. Sci. 2005, 96, 808–813. [Google Scholar] [CrossRef]
- Liang, S.; Liu, L.; Huang, Q.; Yam, K.L. Preparation of single or double-network chitosan/poly(vinyl alcohol) gel films through selectively cross-linking method. Carbohydr. Polym. 2009, 77, 718–724. [Google Scholar] [CrossRef]
- Yamada, M.; Honma, I. Anhydrous proton conductive membrane consisting of chitosan. Electrochim. Acta 2005, 50, 2837–2841. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Q.; Finch, J.A. Silanation and stability of 3-aminopropyl triethoxy silane on nanosized superparamagnetic particles: I. Direct silanation. Appl. Surf. Sci. 1997, 120, 269–278. [Google Scholar] [CrossRef]
- Rueda, L.; Saralegui, A.; Fernández d’Arlas, B.; Zhou, Q.; Berglund, L.A.; Corcuera, M.A.; Eceiza, M.A. Cellulose nanocrystals/polyurethane nanocomposites study from the viewpoint of microphase separated structure. Carbohydr. Polym. 2013, 92, 751–757. [Google Scholar] [CrossRef]
- Ma, X.Y.; Zhang, W.D. Effects of flower-like ZnO nanowhiskers on the mechanical, thermal and antibacterial properties of waterborne polyurethane. Polym. Degrad. Stab. 2009, 94, 1103–1109. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Matterial Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Xu, J.C.; Liu, W.M.; Li, H.L. Titanium dioxide doped polyaniline. Mater. Sci. Eng. 2005, 25, 444–447. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Khan, S.; Park, J.K. Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose 2014, 21, 433–447. [Google Scholar]
- Qin, Y.M.; Zhu, C.J.; Chen, J.; Chen, Y.Z.; Zhang, C. The absorption and release of silver and zinc ions by chitosan fibers. J. Appl. Polym. Sci. 2006, 101, 766–771. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Sunada, K.; Iyoda, T.; Hashimoto, K.; Fujishima, A. Photocatalytic bactericidal effect of TiO2 thin films: Fynamic view of the active oxygen species responsible for the effect. J. Photochem. Photobiol. A 1997, 106, 51–56. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Mahdavi, M.; Abdolmohammadi, S. Preparation, characterization, and antimicrobial activities of ZnO nanoparticles/cellulose nanocrystal nanocomposites. Bioresources 2013, 8, 1841–1851. [Google Scholar]
- Costa-Júnior, E.S.; Barbosa-Stancioli, E.F.; Mansur, A.A.P.; Vasconcelos, W.L.; Mansur, H.S. Preparation and characterization of chitosan/poly(vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohydr. Polym. 2009, 76, 472–481. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( https://creativecommons.org/licenses/by/3.0/).
Share and Cite
Azizi, S.; Ahmad, M.B.; Ibrahim, N.A.; Hussein, M.Z.; Namvar, F. RETRACTED: Cellulose Nanocrystals/ZnO as a Bifunctional Reinforcing Nanocomposite for Poly(vinyl alcohol)/Chitosan Blend Films: Fabrication, Characterization and Properties. Int. J. Mol. Sci. 2014, 15, 11040-11053. https://doi.org/10.3390/ijms150611040
Azizi S, Ahmad MB, Ibrahim NA, Hussein MZ, Namvar F. RETRACTED: Cellulose Nanocrystals/ZnO as a Bifunctional Reinforcing Nanocomposite for Poly(vinyl alcohol)/Chitosan Blend Films: Fabrication, Characterization and Properties. International Journal of Molecular Sciences. 2014; 15(6):11040-11053. https://doi.org/10.3390/ijms150611040
Chicago/Turabian StyleAzizi, Susan, Mansor B. Ahmad, Nor Azowa Ibrahim, Mohd Zobir Hussein, and Farideh Namvar. 2014. "RETRACTED: Cellulose Nanocrystals/ZnO as a Bifunctional Reinforcing Nanocomposite for Poly(vinyl alcohol)/Chitosan Blend Films: Fabrication, Characterization and Properties" International Journal of Molecular Sciences 15, no. 6: 11040-11053. https://doi.org/10.3390/ijms150611040
APA StyleAzizi, S., Ahmad, M. B., Ibrahim, N. A., Hussein, M. Z., & Namvar, F. (2014). RETRACTED: Cellulose Nanocrystals/ZnO as a Bifunctional Reinforcing Nanocomposite for Poly(vinyl alcohol)/Chitosan Blend Films: Fabrication, Characterization and Properties. International Journal of Molecular Sciences, 15(6), 11040-11053. https://doi.org/10.3390/ijms150611040





