2,2',2''-Terpyridine-Catalyzed Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide under Solvent-Free Conditions
Abstract
:1. Introduction
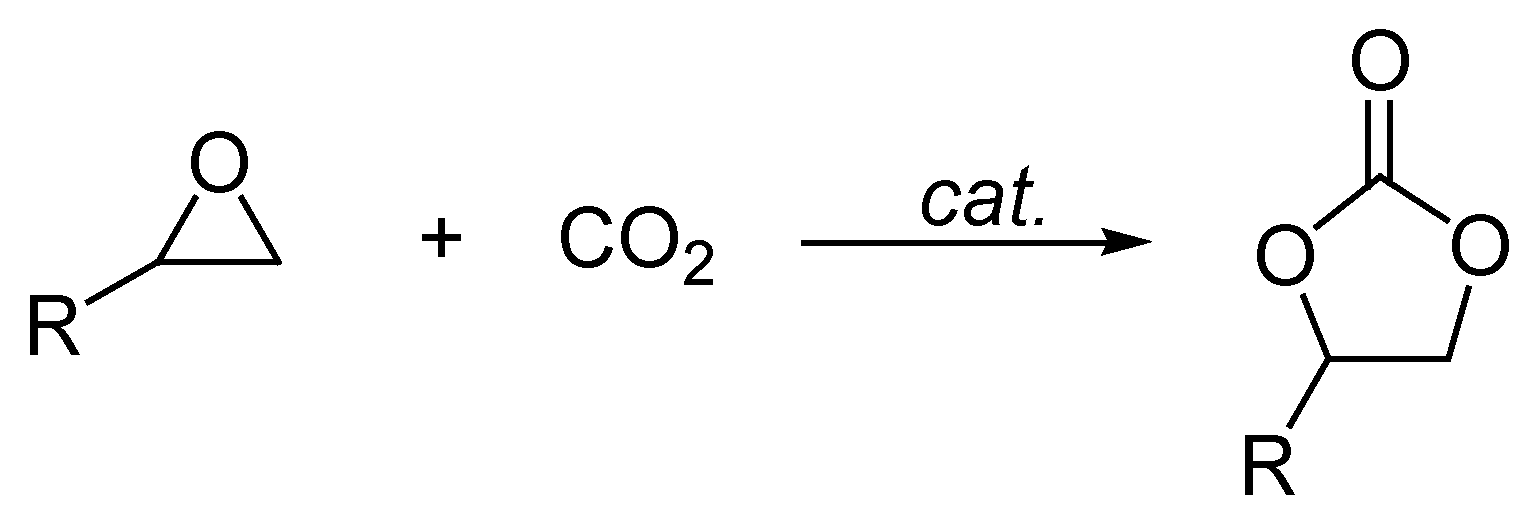
2. Results and Discussion
 | |||||
|---|---|---|---|---|---|
| Entry | Organocatalyst | Yield (%) b | Entry | Organocatalyst | Yield (%) b |
| 1 | Et3N | 48 | 12 | F5C6CONH2 | 71 |
| 2 | Bu3N | 53 | 13 | pyridine | 59 |
| 3 | TEMED | 35 | 14 | 2-methylpyridine | 63 |
| 4 | DBU | 32 | 15 |  | 71 |
| 5 | DABCO | 46 | |||
| 6 | TBD | 56 | 16 |  | 79 |
| 7 | PhNH2 | 59 | |||
| 8 | PhNHMe | 70 | |||
| 9 | PhNMe2 | 81 | 17 |  | 88 |
| 10 | PhCONH2 | 70 | |||
| 11 | PhCONMe2 | 74 | |||
| Entry | Temp (°C) | Catalyst (mol %) | Time (h) | Pressure (MPa) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | 110 | 10 | 20 | 3.0 | 72 |
| 2 | 130 | 5 | 20 | 3.0 | 88 |
| 3 | 130 | 1 | 20 | 3.0 | 87 |
| 4 | 130 | 0.5 | 20 | 3.0 | 68 |
| 5 | 130 | 1 | 10 | 3.0 | 90 |
| 6 | 130 | 1 | 6 | 3.0 | 80 |
| 7 | 130 | 1 | 10 | 2.5 | 81 |
| 8 | 130 | 1 | 10 | 4.0 | 89 |
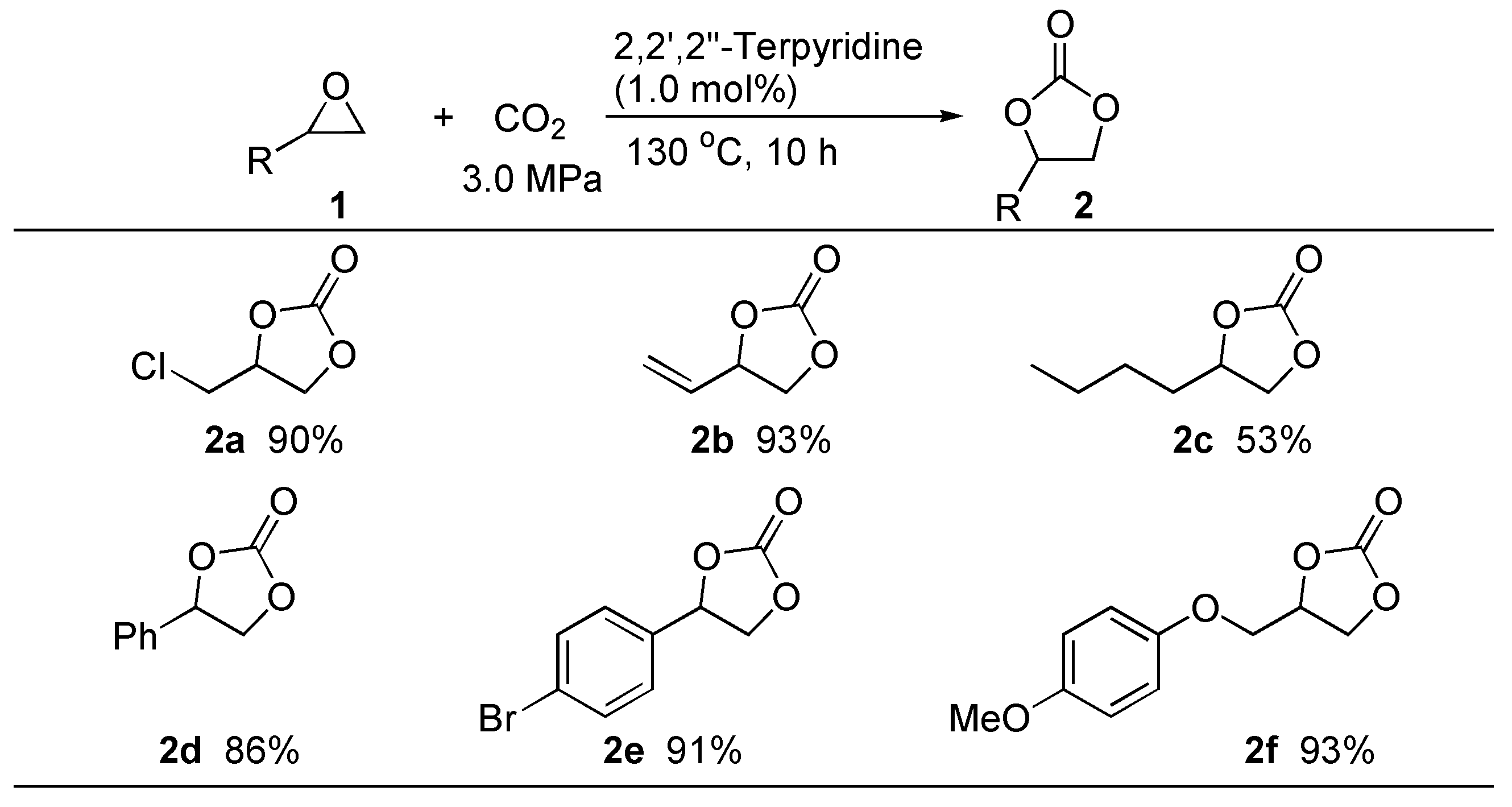
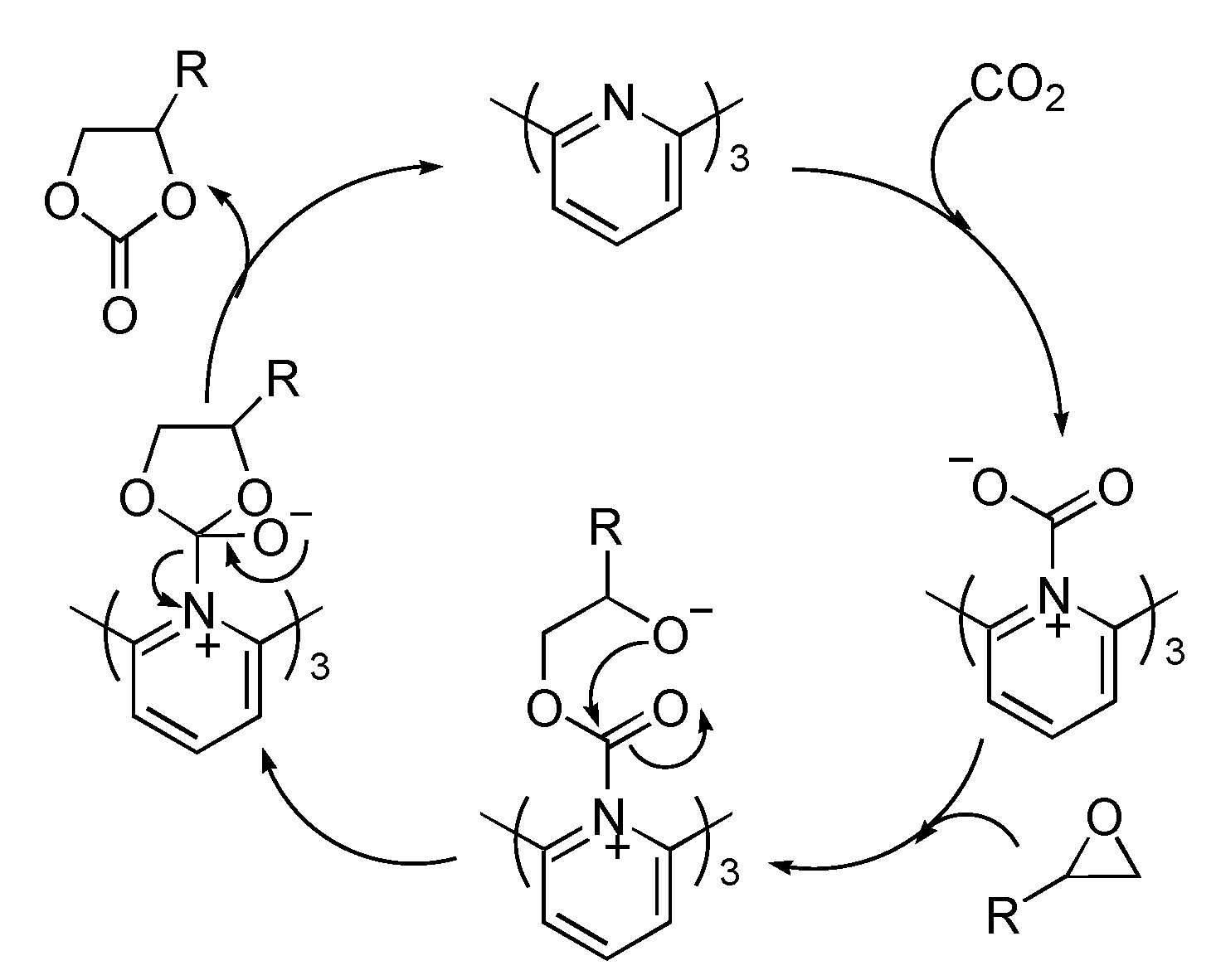
3. Experimental Section
3.1. General Methods
3.2. A Typical Experiment for the Synthesis of 4-Chloromethyl-[1,3]dioxolan-2-one (2a)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bayardon, J.; Holz, J.; Schäffner, B.; Andrushko, V.; Verevkin, S.; Preetz, A.; Börner, A. Propylene carbonate as a solvent for asymmetric hydrogenations. Angew. Chem. 2007, 46, 5971–5974. [Google Scholar] [CrossRef]
- Tsuda, T.; Kondo, K.; Tomioka, T.; Takahashi, Y.; Matsumoto, H.; Kuwabata, S.; Hussey, C.L. Design, synthesis, and electrochemistry of room-temperature ionic liquids functionalized with propylene carbonate. Angew. Chem. 2011, 50, 1310–1313. [Google Scholar] [CrossRef]
- Shaikh, A.A.G.; Sivaram, S. Organic carbonates. Chem. Rev. 1996, 96, 951–976. [Google Scholar]
- Lu, X.B.; Darensbourg, D.J. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Wildeson, J.R.; Yarbrough, J.C.; Reibenspies, J.H. Bis 2,6-difluorophenoxide dimeric complexes of zinc and cadmium and their phosphine adducts: Lessons learned relative to carbon dioxide/cyclohexene oxide alternating copolymerization processes catalyzed by zinc phenoxides. J. Am. Chem. Soc. 2000, 122, 12487–12496. [Google Scholar] [CrossRef]
- Allen, S.D.; Moore, D.R.; Lobkovsky, E.B.; Coates, G.W. High-activity, single-site catalysts for the alternating copolymerization of CO2 and propylene oxide. J. Am. Chem. Soc. 2002, 124, 14284–14285. [Google Scholar] [CrossRef]
- Huang, J.W.; Shi, M. Chemical fixation of carbon dioxide by NaI/PPh3/PhOH. J. Org. Chem. 2003, 68, 6705–6709. [Google Scholar] [CrossRef]
- Peng, J.; Deng, Y. Cycloaddition of carbon dioxide to propylene oxide catalyzed by ionic liquids. New J. Chem. 2001, 25, 639–641. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Fanizzi, A. Cyclic carbonate formation from carbon dioxide and oxiranes in tetrabutylammonium halides as solvents and catalysts. Org. Lett. 2002, 4, 2561–2563. [Google Scholar] [CrossRef]
- Kawanami, H.; Sasaki, A.; Matsui, K.; Ikushima, Y. A rapid and effective synthesis of propylene carbonate using a supercritical CO2-ionic liquid system. Chem. Commun. 2003, 896–897. [Google Scholar]
- Li, F.; Xia, C.; Xu, L.; Sun, W.; Chen, G. A novel and effective Ni complex catalyst system for the coupling reactions of carbon dioxide and epoxides. Chem. Commun. 2003, 2042–2043. [Google Scholar]
- Shen, Y.M.; Duan, W.L.; Shi, M. Chemical fixation of carbon dioxide catalyzed by binaphthyldiamino Zn, Cu, and Co salen-type complexes. J. Org. Chem. 2003, 68, 1559–1562. [Google Scholar]
- Yoshida, M.; Ihara, M. Novel methodologies for the synthesis of cyclic carbonates. Chem. Eur. J. 2004, 10, 2886–2891. [Google Scholar] [CrossRef]
- Jiang, J.L.; Gao, F.; Hua, R.; Qiu, X. Re(CO)5Br-catalyzed coupling of epoxides with CO2 affording cyclic carbonates under solvent-free conditions. J. Org. Chem. 2005, 70, 381–384. [Google Scholar] [CrossRef]
- Decortes, A.; Castilla, A.M.; Kleij, A.W. Salen-complex-mediated formation of cyclic carbonates by cycloaddition of CO2 to epoxides. Angew. Chem. 2010, 49, 9829–9837. [Google Scholar]
- Du, Y.; Wang, J.Q.; Chen, J.Y.; Cai, F.; Tian, J.S.; Kong, D.L.; He, L.N. A poly(ethylene glycol)-supported quaternary ammonium salt for highly efficient and environmentally friendly chemical fixation of CO2 with epoxides under supercritical conditions. Tetrahedron Lett. 2006, 47, 1271–1275. [Google Scholar] [CrossRef]
- Dou, X.Y.; Wang, J.Q.; Du, Y.; Wang, E.; He, L.N. Guanidium salt functionalized PEG: An effective and recyclable homogeneous catalyst for the synthesis of cyclic carbonates from CO2 and epoxides under solvent-free conditions. Synlett 2007, 2007, 3058–3062. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Z.; Jiang, T.; He, J.; Han, B.; Wu, T.; Ding, K. CO2 cycloaddition reactions catalyzed by an ionic liquid grafted onto a highly cross-linked polymer matrix. Angew. Chem. 2007, 46, 7255–7258. [Google Scholar] [CrossRef]
- Dai, W.L.; Chen, L.; Yin, S.F.; Li, W.-H.; Zhang, Y.Y.; Luo, S.L.; Au, C.T. High-efficiency synthesis of cyclic carbonates from epoxides and CO2 over hydroxyl ionic liquid catalyst grafted onto cross-linked polymer. Catal. Lett. 2010, 137, 74–80. [Google Scholar] [CrossRef]
- Bertelsen, S.; Jørgensen, K. A. Organocatalysis—After the gold rush. Chem. Soc. Rev. 2009, 38, 2178–2189. [Google Scholar] [CrossRef]
- Alemán, J.; Cabrera, S. Applications of asymmetric organocatalysis in medicinal chemistry. Chem. Soc. Rev. 2013, 42, 774–793. [Google Scholar] [CrossRef]
- Aoyagi, N.; Furusho, Y.; Sei, Y.; Endo, T. Fast equilibrium of zwitterionic adduct formation in reversible fixationprelease system of CO2 by amidines under dry conditions. Tetrahedron 2013, 69 and references cited therein., 5476–5480. [Google Scholar] [CrossRef]
- Wang, J.Q.; Dong, K.; Cheng, W.G.; Sun, J.; Zhang, S.J. Insights into quaternary ammonium salts-catalyzed fixation carbon dioxide with epoxides. Catal. Sci. Technol. 2012, 2 and references cited therein., 1480–1484. [Google Scholar] [CrossRef]
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Morganstewart, H.; Tsang, S.C. Catalytic coupling of CO2 with epoxide over supported and unsupported amines. J. Phys. Chem. A 2010, 114, 3863–3872. [Google Scholar]
- Jiang, J.L.; Hua, R. Efficient DMF-catalyzed coupling of epoxides with CO2 under solvent-free conditions to afford cyclic carbonates. Synth. Commun. 2006, 36, 3141–3148. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, H.; Zeng, R.; Hua, R. 2,2',2''-Terpyridine-Catalyzed Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide under Solvent-Free Conditions. Int. J. Mol. Sci. 2014, 15, 9945-9951. https://doi.org/10.3390/ijms15069945
Liu H, Zeng R, Hua R. 2,2',2''-Terpyridine-Catalyzed Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide under Solvent-Free Conditions. International Journal of Molecular Sciences. 2014; 15(6):9945-9951. https://doi.org/10.3390/ijms15069945
Chicago/Turabian StyleLiu, Huixin, Renqin Zeng, and Ruimao Hua. 2014. "2,2',2''-Terpyridine-Catalyzed Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide under Solvent-Free Conditions" International Journal of Molecular Sciences 15, no. 6: 9945-9951. https://doi.org/10.3390/ijms15069945





