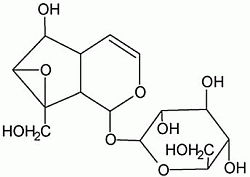
Catalpol Ameliorates Sodium Taurocholate-Induced Acute Pancreatitis in Rats via Inhibiting Activation of Nuclear Factor Kappa B
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Preliminary Study

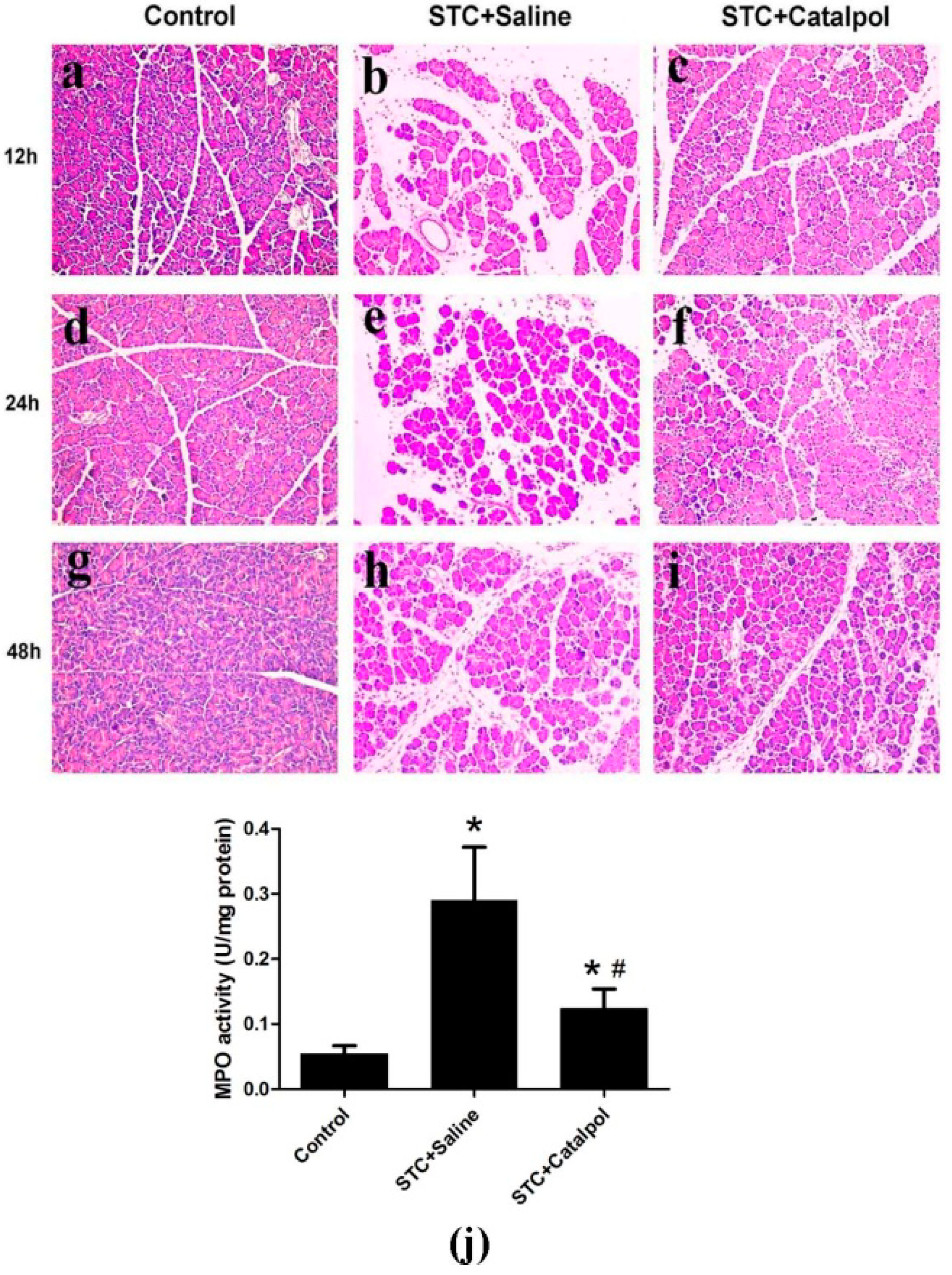
2.1.2. Effect of Catalpol on STC-Induced AP in Vivo




2.1.3. Effect of Catalpol on STC-Induced AP in Vitro

2.2. Discussion
3. Experimental
3.1. Animals and Main Materials
3.2. Induction of Experimental Design
3.3. Isolation of Pancreatic Acinar Cells
3.4. Determination of Serum Amylase, Lipase and Serum Proinflammatory Cytokines
3.5. Histological Examination
3.6. Measurement of Myeloperoxidase Activity
3.7. Quantitative Real-Time PCR
3.8. Western Blot Analysis
3.9. Immunohistochemistry
3.10. Quantification of Cell Viability
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baron, T.H.; Morgan, D.E. Acute necrotizing pancreatitis. N. Engl. J. Med. 1999, 340, 1412–1417. [Google Scholar] [CrossRef]
- Frossard, J.L.; Steer, M.L.; Pastor, C.M. Acute pancreatitis. Lancet 2008, 371, 143–152. [Google Scholar] [CrossRef]
- Bhatia, M. Inflammatory response on the pancreatic acinar cell injury. Scand. J. Surg. 2005, 94, 97–102. [Google Scholar]
- Zhang, X.P.; Jiang, J.; Yu, Y.P.; Cheng, Q.H.; Chen, B. Effect of Danshen on apoptosis and NF-kappaB protein expression of the intestinal mucosa of rats with severe acute pancreatitis or obstructive jaundice. HBPD Int. 2010, 9, 537–546. [Google Scholar]
- Grady, T.; Liang, P.; Ernst, S.A.; Logsdon, C.D. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology. 1997, 113, 1966–1975. [Google Scholar] [CrossRef]
- Gukovsky, I.; Gukovskaya, A.S.; Blinman, T.A.; Zaninovic, V.; Pandol, S.J. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am. J. Physiol. 1998, 275, G1402–G1414. [Google Scholar]
- Satoh, A.; Shimosegawa, T.; Fujita, M.; Kimura, K.; Masamune, A.; Koizumi, M.; Toyota, T. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut 1999, 44, 253–258. [Google Scholar] [CrossRef]
- Rakonczay, Z., Jr.; Hegyi, P.; Takacs, T.; McCarroll, J.; Saluja, A.K. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut 2008, 57, 259–267. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Y.; Daniluk, J.; Gaiser, S.; Chu, J.; Wang, H.; Li, Z.S.; Logsdon, C.D.; Ji, B. Activation of nuclear factor-kappaB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology 2013, 144, 202–210. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.G.; He, X.W.; Li, X.R.; Din, B.N.; Gan, Y.; Xu, M. Hyperbaric oxygen reduces inflammatory response in acute pancreatitis by inhibiting NF-kappaB activation. Eur. Surg. Res. 2009, 42, 130–135. [Google Scholar] [CrossRef]
- Ismailoglu, U.B.; Saracoglu, I.; Harput, U.S.; Sahin-Erdemli, I. Effects of phenylpropanoid and iridoid glycosides on free radical-induced impairment of endothelium-dependent relaxation in rat aortic rings. J. Ethnopharmacol. 2002, 79, 193–197. [Google Scholar] [CrossRef]
- Pungitore, C.R.; Leon, L.G.; Garcia, C.; Martin, V.S.; Tonn, C.E.; Padron, J.M. Novel antiproliferative analogs of the Taq DNA polymerase inhibitor catalpol. Bioorg. Med. Chem. Lett. 2007, 17, 1332–1335. [Google Scholar]
- Tian, Y.Y.; An, L.J.; Jiang, L.; Duan, Y.L.; Chen, J.; Jiang, B. Catalpol protects dopaminergic neurons from LPS-induced neurotoxicity in mesencephalic neuron-glia cultures. Life Sci. 2006, 80, 193–199. [Google Scholar] [CrossRef]
- Jiang, B.; Du, J.; Liu, J.H.; Bao, Y.M.; An, L.J. Catalpol attenuates the neurotoxicity induced by beta-amyloid(1–42) in cortical neuron-glia cultures. Brain Res. 2008, 1188, 139–147. [Google Scholar]
- Kim, B.H.; Park, K.S.; Chang, I.M. Elucidation of anti-inflammatory potencies of Eucommia ulmoides bark and Plantago asiatica seeds. J. Med. Food 2009, 12, 764–769. [Google Scholar] [CrossRef]
- Zhang, A.; Hao, S.; Bi, J.; Bao, Y.; Zhang, X.; An, L.; Jiang, B. Effects of catalpol on mitochondrial function and working memory in mice after lipopolysaccharide-induced acute systemic inflammation. Exp. Toxicol. Pathol. 2009, 61, 461–469. [Google Scholar] [CrossRef]
- Bi, J.; Jiang, B.; Zorn, A.; Zhao, R.G.; Liu, P.; An, L.J. Catalpol inhibits LPS plus IFN-γ-induced inflammatory response in astrocytes primary cultures. Toxicol. In Vitro 2013, 27, 543–550. [Google Scholar] [CrossRef]
- Wan, M.H.; Huang, W.; Latawiec, D.; Jiang, K.; Booth, D.M.; Elliott, V.; Mukherjee, R.; Xia, Q. Review of experimental animal models of biliary acute pancreatitis and recent advances in basic research. HPB (Oxford) 2012, 14, 73–81. [Google Scholar] [CrossRef]
- Fortunato, F.; Burgers, H.; Bergmann, F.; Rieger, P.; Buchler, M.W.; Kroemer, G.; Werner, J. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology 2009, 137, 350–360. [Google Scholar] [CrossRef]
- Pandol, S.J.; Saluja, A.K.; Imrie, C.W.; Banks, P.A. Acute pancreatitis: bench to the bedside. Gastroenterology 2007, 132, 1127–1151. [Google Scholar] [CrossRef]
- Heath, D.I.; Cruickshank, A.; Gudgeon, M.; Jehanli, A.; Shenkin, A.; Imrie, C.W. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut 1993, 34, 41–45. [Google Scholar] [CrossRef]
- Steinberg, W.; Tenner, S. Acute pancreatitis. N. Engl. J. Med. 1994, 330, 1198–1210. [Google Scholar] [CrossRef]
- Beger, H.G.; Rau, B.; Mayer, J.; Pralle, U. Natural course of acute pancreatitis. World J. Surg. 1997, 21, 130–135. [Google Scholar] [CrossRef]
- Kaiser, A.M.; Saluja, A.K.; Steer, M.L. Repetitive short-term obstructions of the common bile-pancreatic duct induce severe acute pancreatitis in the opossum. Dig. Dis. Sci. 1999, 44, 1653–1661. [Google Scholar] [CrossRef]
- Bhatia, M.; Brady, M.; Shokuhi, S.; Christmas, S.; Neoptolemos, J.P.; Slavin, J. Inflammatory mediators in acute pancreatitis. J. Pathol. 2000, 190, 117–125. [Google Scholar] [CrossRef]
- Gross, V.; Leser, H.G.; Heinisch, A.; Scholmerich, J. Inflammatory mediators and cytokines—New aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology 1993, 40, 522–530. [Google Scholar]
- Bae, G.S.; Kim, M.S.; Jeong, J.; Lee, H.Y.; Park, K.C.; Koo, B.S.; Kim, B.J.; Kim, T.H.; Lee, S.H.; Hwang, S.Y.; et al. Piperine ameliorates the severity of cerulein-induced acute pancreatitis by inhibiting the activation of mitogen activated protein kinases. Biochem. Biophys. Res. Commun. 2011, 410, 382–388. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhang, L.; Chen, L.J.; Cheng, Q.H.; Wang, J.M.; Cai, W.; Shen, H.P.; Cai, J. Influence of dexamethasone on inflammatory mediators and NF-kappaB expression in multiple organs of rats with severe acute pancreatitis. World J. Gastroenterol. 2007, 13, 548–556. [Google Scholar] [CrossRef]
- Wang, G.; Sun, B.; Zhu, H.; Gao, Y.; Li, X.; Xue, D.; Jiang, H. Protective effects of emodin combined with danshensu on experimental severe acute pancreatitis. Inflamm. Res. 2010, 59, 479–488. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, C.; Li, Y.; Guan, S.; Han, F.; Zhang, S. Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by d-galactose. Food Chem. Toxicol. 2013, 58, 50–55. [Google Scholar] [CrossRef]
- Bi, J.; Jiang, B.; Hao, S.; Zhang, A.; Dong, Y.; Jiang, T.; An, L. Catalpol attenuates nitric oxide increase via ERK signaling pathways induced by rotenone in mesencephalic neurons. Neurochem. Int. 2009, 54, 264–270. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, J.H.; Bao, Y.M.; An, L.J. Catalpol inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Toxicon 2004, 43, 53–59. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Hu, J. Catalpol inhibits apoptosis in hydrogen peroxide-induced endothelium by activating the PI3K/Akt signaling pathway and modulating expression of Bcl-2 and Bax. Eur. J. Pharmacol. 2010, 628, 155–163. [Google Scholar] [CrossRef]
- Choi, H.J.; Jang, H.J.; Chung, T.W.; Jeong, S.I.; Cha, J.; Choi, J.Y.; Han, C.W.; Jang, Y.S.; Joo, M.; Jeong, H.S.; et al. Catalpol suppresses advanced glycation end-products-induced inflammatory responses through inhibition of reactive oxygen species in human monocytic THP-1 cells. Fitoterapia 2013, 86, 19–28. [Google Scholar]
- Neurath, M.F.; Becker, C.; Barbulescu, K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut 1998, 43, 856–860. [Google Scholar] [CrossRef]
- Chen, X.; Ji, B.; Han, B.; Ernst, S.A.; Simeone, D.; Logsdon, C.D. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology 2002, 122, 448–457. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, B.; Zhang, X.; Zhang, L.; Bai, X.; Zhao, X.; Chen, L.; Cui, L.; Zhu, C.; Wang, L.; et al. Bicyclol upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. Bull. 2014, 100, 38–43. [Google Scholar] [CrossRef]
- Ni, J.; Hu, G.; Xiong, J.; Shen, J.; Yang, L.; Tang, M.; Zhao, Y.; Ying, G.; Yu, G.; Hu, Y.; et al. Involvement of interleukin-17A in pancreatic damage in rat experimental acute necrotizing pancreatitis. Inflammation 2013, 36, 53–65. [Google Scholar] [CrossRef]
- Hu, G.; Shen, J.; Cheng, L.; Guo, C.; Xu, X.; Wang, F.; Huang, L.; Yang, L.; He, M.; Xiang, D.; et al. Reg4 protects against acinar cell necrosis in experimental pancreatitis. Gut 2011, 60, 820–828. [Google Scholar] [CrossRef]
- Ethridge, R.T.; Chung, D.H.; Slogoff, M.; Ehlers, R.A.; Hellmich, M.R.; Rajaraman, S.; Saito, H.; Uchida, T.; Evers, B.M. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology 2002, 123, 1311–1322. [Google Scholar] [CrossRef]
- Van Acker, G.J.; Saluja, A.K.; Bhagat, L.; Singh, V.P.; Song, A.M.; Steer, M.L. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G794–800. [Google Scholar]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef]
Appendix

© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiao, W.Q.; Yin, G.J.; Fan, Y.T.; Qiu, L.; Cang, X.F.; Yu, G.; Hu, Y.L.; Xing, M.; Wu, D.Q.; Wang, X.P.; et al. Catalpol Ameliorates Sodium Taurocholate-Induced Acute Pancreatitis in Rats via Inhibiting Activation of Nuclear Factor Kappa B. Int. J. Mol. Sci. 2014, 15, 11957-11972. https://doi.org/10.3390/ijms150711957
Xiao WQ, Yin GJ, Fan YT, Qiu L, Cang XF, Yu G, Hu YL, Xing M, Wu DQ, Wang XP, et al. Catalpol Ameliorates Sodium Taurocholate-Induced Acute Pancreatitis in Rats via Inhibiting Activation of Nuclear Factor Kappa B. International Journal of Molecular Sciences. 2014; 15(7):11957-11972. https://doi.org/10.3390/ijms150711957
Chicago/Turabian StyleXiao, Wen Qin, Guo Jian Yin, Yu Ting Fan, Lei Qiu, Xiao Feng Cang, Ge Yu, Yan Ling Hu, Miao Xing, De Qing Wu, Xing Peng Wang, and et al. 2014. "Catalpol Ameliorates Sodium Taurocholate-Induced Acute Pancreatitis in Rats via Inhibiting Activation of Nuclear Factor Kappa B" International Journal of Molecular Sciences 15, no. 7: 11957-11972. https://doi.org/10.3390/ijms150711957