Calcium and Zinc Containing Bactericidal Glass Coatings for Biomedical Metallic Substrates
Abstract
:1. Introduction
2. Results
2.1. Coating Characterization
| α (10−6·K−1) | ||||||
|---|---|---|---|---|---|---|
| ZnO15 | ZnO35 | G3 | Ti Alloy | Ta | Nb | Stainless Steel 316L |
| 7.7 | 10.7 | 14.2 | 9.7 | 6.3 | 7.3 | 16.0 |






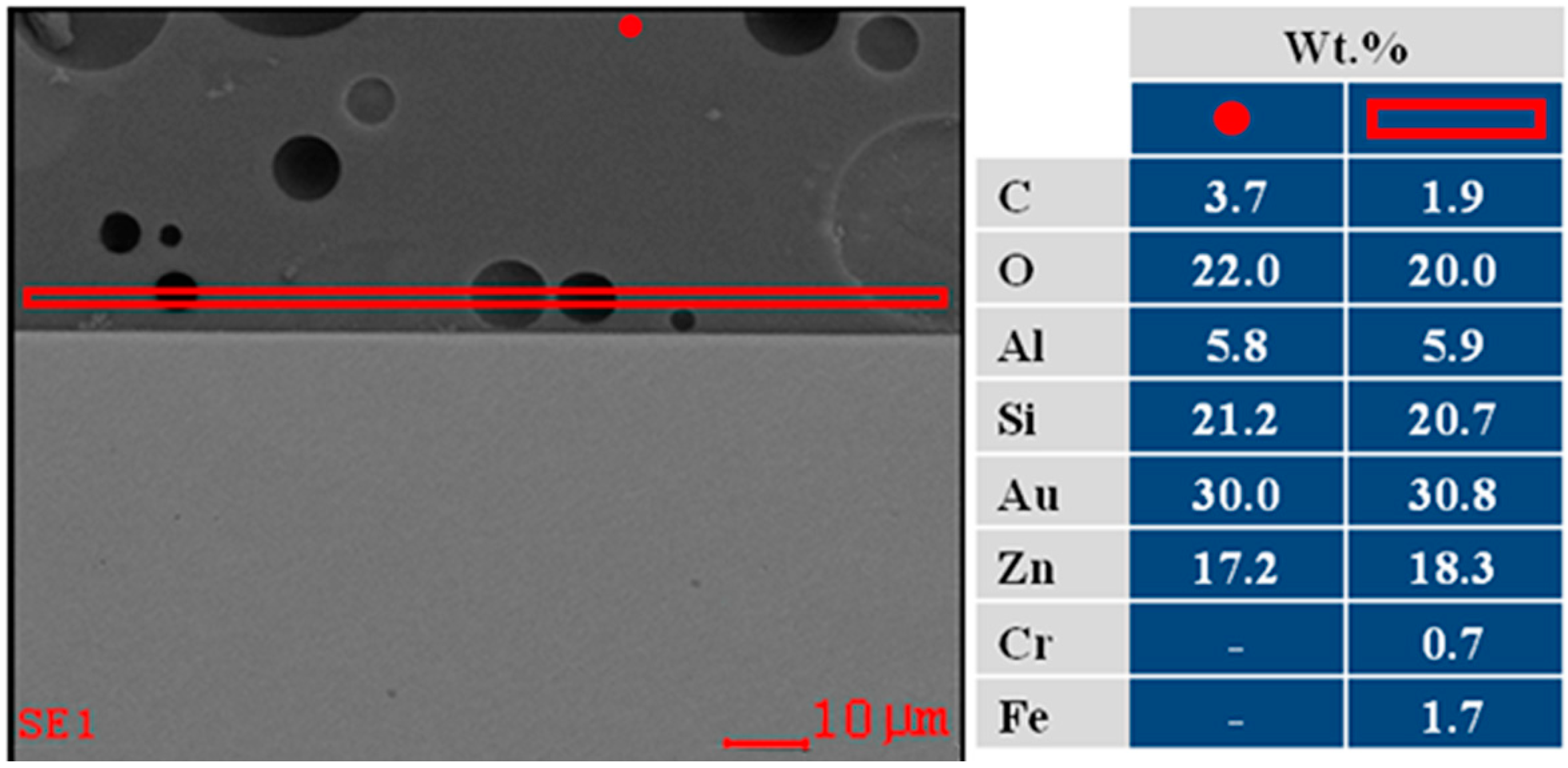
2.2. Bactericidal Activity

3. Discussion



| Metal | Δ G°1026.8 °C for Reactions 1 and 7 (kcal/mol) | Δ G°1026.8 °C for Reactions 3 and 9 (kcal/mol) |
|---|---|---|
| Fe | −89.9 | 65.5 |
| Ta | −141.6 | 198.3 |
| Nb/Nb2+ | −144.6 | 38.1 |
| Nb/Nb5+ | −128.0 | 232.4 |
| Ti/Ti2+ | −200.2 | 10.4 |
| Ti/Ti3+ | −185.5 | 53.1 |
| Ti/Ti4+ | −168.2 | 52.8 |
4. Experimental Section
4.1. Materials
| Glass | SiO2 | B2O3 | Na2O | CaO | Al2O3 | K2O | ZnO | Th | Tg | Ts |
|---|---|---|---|---|---|---|---|---|---|---|
| ZnO15 | 29.4 | 45.0 | 8.2 | – | 4.6 | – | 12.8 | 715 | 487 | 513 |
| ZnO35 | 23.1 | 35.3 | 6.4 | – | 3.6 | – | 31.6 | 666 | 475 | 510 |
| G3 | 43.0 | 7.8 | 19.4 | 22.0 | 7.4 | 0.4 | – | 934 | 507 | 535 |
4.2. Preparation of the Coatings
4.3. Coatings Characterization
4.4. Bactericidal Test
5. Conclusions
- Ti6Al4V and stainless steel 316L in air;
- Ti6Al4V, Ta, and Nb in argon atmosphere.
- Stainless steel 316L in air;
- Ta, and Nb in argon atmosphere.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Park, J.B.; Bronzino, J.D. Biomaterials: Principles and Applications; CRC Press: Boca Ratón, FL, USA, 2003; pp. 1–241. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Wise, D.L. Biomaterials Engineering and Devices; Humana Press: Berlín, Germany, 2000; pp. 205–319. [Google Scholar]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement: A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Ha, J.Y.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Kim, K.H.; Hanawa, T. Enhancement of calcium phosphate formation on zirconium by micro-arc oxidation and chemical treatments. Surf. Coat. Technol. 2011, 205, 4948–4955. [Google Scholar] [CrossRef]
- Spriano, S.; Ferraris, S. Metallic surfaces for osteointegration. In Surface Tailoring of Inorganic Materials for Biomedical Applications; Rimondini, L., Ed.; Bentham Science Publishers Ltd.: Oak Park, IL, USA, 2012; pp. 279–296. [Google Scholar]
- Rabih, O.; Darouiche, M.D. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Lyndon, J.; Boyd, B.; Birbilis, N. Metallic implant drug/device combinations for controlled drug release in orthopaedic applications. J. Control. Release 2014, 179, 63–75. [Google Scholar] [CrossRef]
- Matl, F.D.; Obermeier, A.; Repmann, S.; Friess, W.; Stemberger, A.; Kuehn, K.D. New anti-infective coatings of medical implants. Antimicrob. Agents Chemother. 2008, 52, 1957–1963. [Google Scholar] [CrossRef]
- Antoci, V.; King, S.B.; Jose, B.; Parvizi, J.; Zeiger, A.R.; Wickstrom, E.; Freeman, T.A.; Composto, R.J.; Ducheyne, P.; Shapiro, I.M.; et al. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J. Orthop. Res. 2007, 25, 858–866. [Google Scholar] [CrossRef]
- Jose, B.; Antoci, V., Jr.; Zeiger, A.R.; Wickstrom, E.; Hickok, N.J. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem. Biol. 2005, 12, 1041–1048. [Google Scholar] [CrossRef]
- Mani, G.; Johnson, D.M.; Marton, D.; Feldman, M.D.; Patel, D.; Ayon, A.A.; Agrawal, C.M. Drug delivery from gold and titanium surfaces using self-assembled monolayers. Biomaterials 2008, 29, 4561–4573. [Google Scholar] [CrossRef]
- Esteban-Tejeda, L.; Cabal, B.; Malpartida, F.; López-Piriz, R.; Torrecillas, R.; Saiz, E.; Tomsia, A.P.; Moya, J.S. Soda-Lime glass coating containing silver nanoparticles on Ti-6Al-4V alloy. J. Eur. Ceram. Soc. 2012, 32, 2723–2729. [Google Scholar] [CrossRef]
- López Píriz, R.; Solá Linares, E.; Granizo, J.; Díaz Güemes, I.; Enciso, S.; Bartolomé, J.; Cabal, B.; Esteban Tejeda, L.; Torrecillas, R.; Moya, J.; et al. Radiologic evaluation of bone loss at implants with biocide coated titanium abutments: A study in the dog. PLoS One 2012, 7, e52861. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, J.; Liu, X.; Sun, J. Antimicrobial and osteogenic effect of Ag-implanted titanium with a nanostructured surface. Int. J. Nanomed. 2012, 7, 875–884. [Google Scholar]
- Esteban-Tejeda, L.; Prado, C.; Cabal, B.; Sanz, J.; Torrecillas, R.; Moya, J.S. Antibacterial and antifungal activity of ZnO non-toxic and environmentally friendly glasses. Environ. Sci. Technol. 2014, in press. [Google Scholar]
- Brayner, R.; Ferrari Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Huh, A.; Kwon, Y. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef]
- Jiang, P.; Zhou, J.J.; Fang, H.F.; Wang, C.Y.; Wang, Z.L.; Xie, S.S. Hierarchical shelled ZnO structures made of bunched nanowire arrays. Adv. Funct. Mater. 2007, 17, 1303–1310. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, N.S.; Wang, Z.L. Dissolving behavior and stability of ZnO wires in biofluids: A study on biodegradability and biocompatibility of ZnO nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
- Moya, J.S.; Esteban-Tejeda, L.; Pecharromán, C.; Mello-Castanho, S.R.H.; da Silva, A.C.; Malpartida, F. Glass-powders with a high content of calcium oxide: A step toward a “Green” universal biocide. Adv. Biomater. 2011, 64, 256–260. [Google Scholar]
- Cabal, B.; Alou, L.; Cafini, F.; Couceiro, R.; Sevillano, D.; Esteban-Tejeda, L.; Guitián, F.; Torrecillas, R.; Moya, J.S. A new biocompatible and antibacterial phosphate free glass-ceramic for medical applications. Sci. Rep. 2014. [Google Scholar] [CrossRef]
- Esteban Tejeda, L.; Díaz, L.A.; Cabal, B.; Prado, C.; López Piriz, R.; Torrecillas, R.; Moya, J.S. Biocide glass–ceramic coating on titanium alloy and zirconium oxide for dental applications. Mater. Lett. 2013, 111, 59–62. [Google Scholar] [CrossRef]
- Bellantone, M.; William, H.D.; Hench, L.L. Broad-spectrum bactericidal activity of Ag2O-doped bioactive glass. Antimicrob. Agent Chemother. 2002, 46, 1940–1945. [Google Scholar] [CrossRef]
- Verné, E.; Nunzio, S.D.; Bosetti, M.; Appendino, P.; Vitale Brovarone, C.; Maina, G.; Cannasb, M. Surface characterization of silver-doped bioactive glass. Biomaterials 2005, 26, 5111–5119. [Google Scholar] [CrossRef]
- Aina, V.; Malavasi, G.; Fiorio Pla, A.; Munaron, L.; Morterra, C. Zinc-containing bioactive glasses: Surface reactivity and behaviour towards endothelial cells. Acta Biomater. 2009, 5, 1211–1222. [Google Scholar] [CrossRef]
- Verne, E.; Verné, E.; Ferraris, S.; Miola, M.; Fucale, G.; Maina, G.; Robotti, P.; Bianchi, G.; Martinasso, G.; Canuto, R.A.; et al. Synthesis and characterisation of bioactive and antibacterial glass-ceramic Part 2—Plasma spray coatings on metallic substrates. Adv. Appl. Ceram. 2008, 107, 245–253. [Google Scholar] [CrossRef]
- Addison, O.; Davenport, A.J.; Newport, R.J.; Kalra, S.; Monir, M.; Mosselmans, J.F.W.; Proops, D.; Martin, R.A. Do “passive” medical titanium surfaces deteriorate in service in the absence of wear? J. R. Soc. Interface 2012, 9, 3161–3164. [Google Scholar] [CrossRef]
- Pazo, A.; Saiz, E.; Tomsia, A.P. Silicate glass coatings on Ti-based implants. Acta Mater. 1998, 46, 2551–2558. [Google Scholar] [CrossRef]
- Tomsia, A.P.; Pask, J.A. Chemical reactions and adherence at glass/metal interfaces: An analysis. Dent. Mater. 1986, 2, 10–16. [Google Scholar] [CrossRef]
- Yousef, J.M.; Daniel, E.N. In vitro antibacterial activity and minimum inhibitory concentration of zinc oxide and nano-particle zinc oxide against pathogenic strains. J. Health Sci. 2012, 2, 38–42. [Google Scholar] [CrossRef]
- ISO 22196 Standard Method. Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. Available online: http://www.iso.org/ (accessed on Day Month 2011).
- Moriarty, F.Z.; Sebastian, A.J. Biomaterials Associated Infection; Busscher, H.J., Ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Esteban-Tejeda, L.; Díaz, L.A.; Prado, C.; Cabal, B.; Torrecillas, R.; Moya, J.S. Calcium and Zinc Containing Bactericidal Glass Coatings for Biomedical Metallic Substrates. Int. J. Mol. Sci. 2014, 15, 13030-13044. https://doi.org/10.3390/ijms150713030
Esteban-Tejeda L, Díaz LA, Prado C, Cabal B, Torrecillas R, Moya JS. Calcium and Zinc Containing Bactericidal Glass Coatings for Biomedical Metallic Substrates. International Journal of Molecular Sciences. 2014; 15(7):13030-13044. https://doi.org/10.3390/ijms150713030
Chicago/Turabian StyleEsteban-Tejeda, Leticia, Luis A. Díaz, Catuxa Prado, Belén Cabal, Ramón Torrecillas, and José S. Moya. 2014. "Calcium and Zinc Containing Bactericidal Glass Coatings for Biomedical Metallic Substrates" International Journal of Molecular Sciences 15, no. 7: 13030-13044. https://doi.org/10.3390/ijms150713030




