Identification and Analysis of Regulatory Elements in Porcine Bone Morphogenetic Protein 15 Gene Promoter
Abstract
:1. Introduction
2. Results
2.1. Molecular Cloning and Bioinformatics Analysis of Bone Morphogenetic Protein 15 (BMP15) Promoter


2.2. Investigation of Primary Regulatory Elements in BMP15 Promoter


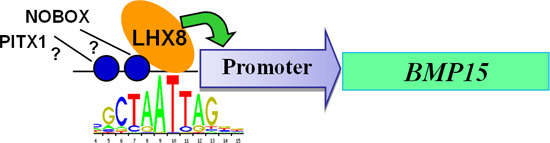
2.3. LIM Homeobox 8 (LHX8) Regulates BMP15 Promoter Activation

3. Discussion
4. Experimental Methods
4.1. Cell Culture
4.2. Gene Cloning and Vector Construction
4.3. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.4. Biological Assays
4.5. Bioinformatics Analysis
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, X.J.; Mei, S.Q.; Tao, H.; Wang, G.D.; Su, L.N.; Jiang, S.W.; Deng, C.Y.; Xiong, Y.Z.; Li, F.G. Microarray profiling for differential gene expression in PMSG-hCG stimulated preovulatory ovarian follicles of Chinese Taihu and Large White sows. BMC Genom. 2011, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- McNatty, K.P.; Moore, L.G.; Hudson, N.L.; Quirke, L.D.; Lawrence, S.B.; Reader, K.; Hanrahan, J.P.; Smith, P.; Groome, N.P.; Laitinen, M.; et al. The oocyte and its role in regulating ovulation rate: A new paradigm in reproductive biology. Reproduction 2004, 128, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Emori, C.; Sugiura, K. Role of oocyte-derived paracrine factors in follicular development. Anim. Sci. J. 2014, 85, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Knight, P.G.; Glister, C. TGF-β superfamily members and ovarian follicle development. Reproduction 2006, 132, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.L.; Li, Y.H.; Xu, Y.N.; Wang, Q.L.; Namgoong, S.; Cui, X.S.; Kim, N.H. Effects of growth differentiation factor 9 and bone morphogenetic protein 15 on the in vitro maturation of porcine oocytes. Reprod. Domest. Anim. 2014, 49, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Ritter, L.J.; Schulz, S.J.; Amato, F.; Thompson, J.G.; Armstrong, D.T.; Gilchrist, R.B. Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol. Reprod. 2007, 76, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Q.; Wigglesworth, K.; Rangarajan, A.; Kattamuri, C.; Peterson, R.T.; Eppig, J.J.; Thompson, T.B.; Matzuk, M.M. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc. Natl. Acad. Sci. USA 2013, 110, E776–E785. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Matzuk, M.M. GDF-9 and BMP-15: Oocyte organizers. Rev. Endocr. Metab. Disord. 2002, 3, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.M.; McNatty, K.P.; Cambridge, L.M.; Laitinen, M.P.E.; Juengel, J.L.; Jokiranta, T.S.; McLaren, R.J.; Luiro, K.; Dodds, K.G.; Montgomery, G.W.; et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat. Genet. 2000, 25, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Demars, J.; Fabre, S.; Sarry, J.; Rossetti, R.; Gilbert, H.; Persani, L.; Tosser-Klopp, G.; Mulsant, P.; Nowak, Z.; Drobik, W.; et al. Genome-wide association studies identify two novel BMP15 mutations responsible for an atypical hyperprolificacy phenotype in sheep. PLoS Genet. 2013, 9, e1003482. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.L.; McNatty, K.P. The ratio of growth differentiation factor 9: Bone morphogenetic protein 15 mRNA expression is tightly co-regulated and differs between species over a wide range of ovulation rates. Mol. Cell. Endocrinol. 2012, 348, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Wei, J.; Yu, X.; Zhang, J.; Liu, X.; Ma, X.; Wang, H. Construction and specificity of porcine Bmp15 gene reporter vector. Sheng Wu Gong Cheng Xue Bao 2014, 30, 203–212. [Google Scholar] [PubMed]
- Persani, L.; Rossetti, R.; di Pasquale, E.; Cacciatore, C.; Fabre, S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum. Reprod. Update 2014, 20, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Margulis, S.; Abir, R.; Felz, C.; Nitke, S.; Krissi, H.; Fisch, B. Bone morphogenetic protein 15 expression in human ovaries from fetuses, girls, and women. Fertil. Steril. 2009, 92, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Li, H.K.; Kuo, T.Y.; Yang, H.S.; Chen, L.R.; Li, S.S.; Huang, H.W. Differential gene expression of bone morphogenetic protein 15 and growth differentiation factor 9 during in vitro maturation of porcine oocytes and early embryos. Anim. Reprod. Sci. 2008, 103, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.J.; Gu, P.; Xu, X.; Jackson, K.J.; DeMayo, F.J.; O’Malley, B.W.; Cooney, A.J. GCNF-dependent repression of BMP-15 and GDF-9 mediates gamete regulation of female fertility. Embo J. 2003, 22, 4070–4081. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.J.; Ortega-Recalde, O.; Esteban-Perez, C.; Moreno-Ortiz, H.; Patino, L.C.; Bermudez, O.M.; Ortiz, A.M.; Restrepo, C.M.; Lucena, E.; Laissue, P. BMP15 c.-9C>G promoter sequence variant may contribute to the cause of non-syndromic premature ovarian failure. Reprod. Biomed. Online 2014, 29, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Jagarlamudi, K.; Rajkovic, A. Oogenesis: Transcriptional regulators and mouse models. Mol. Cell. Endocrinol. 2012, 356, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Griffith, G.J.; Trask, M.C.; Hiller, J.; Walentuk, M.; Pawlak, J.B.; Tremblay, K.D.; Mager, J. Yin-yang1 is required in the mammalian oocyte for follicle expansion. Biol. Reprod. 2011, 84, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.A.; Tjian, R. Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nat. Rev. Genet. 2010, 11, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Mo, D.L.; Li, A.N.; Gong, W.; Zhang, Y.; Qian, W.W.; Chen, W.Q.; Xiao, S.Q.; Chen, Y.S. Characterization and transcriptional regulation analysis of the porcine PAQR6 gene. DNA Cell Biol. 2011, 30, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Mei, S.; Zhang, X.; Peng, X.; Yang, J.; Zhu, L.; Zhou, J.; Wu, H.; Wang, L.; Hua, L.; et al. Transcription factor C/EBPβ and 17β-estradiol promote transcription of the porcine p53 gene. Int. J. Biochem. Cell Biol. 2014, 47, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Tarnawa, E.D.; Baker, M.D.; Aloisio, G.M.; Carr, B.R.; Castrillon, D.H. Gonadal expression of FOXO1, but not FOXO3, is conserved in diverse Mammalian species. Biol. Reprod. 2013, 88, 103. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Ballow, D.J.; Xin, Y.; Rajkovic, A. Lim homeobox gene, LHX8, is essential for mouse oocyte differentiation and survival. Biol. Reprod. 2008, 79, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Puthanveetil, P.; Wan, A.; Rodrigues, B. FOXO1 is crucial for sustaining cardiomyocyte metabolism and cell survival. Cardiovasc. Res. 2013, 97, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Kumar, G.S.; Kadakol, A.; Malek, V.; Gaikwad, A.B. FOXO1 inhibitors: The future medicine for metabolic disorders? Curr. Diabetes Rev. 2015, in press. [Google Scholar]
- Pangas, S.A.; Choi, Y.; Ballow, D.J.; Zhao, Y.G.; Westphal, H.; Matzuk, M.M.; Rajkovic, A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and LHX8. Proc. Natl. Acad. Sci. USA 2006, 103, 8090–8095. [Google Scholar] [CrossRef] [PubMed]
- Abir, R.; Fisch, B. Invited commentary: A single nucleotide polymorphism in BMP15 is associated with high response to controlled ovarian hyperstimulation. Reprod. Biomed. Online 2011, 23, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Hanevik, H.I.; Hilmarsen, H.T.; Skjelbred, C.F.; Tanbo, T.; Kahn, J.A. A single nucleotide polymorphism in BMP15 is associated with high response to ovarian stimulation. Reprod. Biomed. Online 2011, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Ripoll, G.; Joy, M.; Folch, J.; Panea, B.; Calvo, J.H.; Alabart, J.L. Effects of the FecXR allele of BMP15 gene on the birth weight, growth rate and carcass quality of Rasa Aragonesa light lambs. Small Rumin. Res. 2012, 108, 45–53. [Google Scholar] [CrossRef]
- Mester, B.; Ritter, L.J.; Pitman, J.L.; Bibby, A.H.; Gilchrist, R.B.; McNatty, K.P.; Juengel, J.L.; McIntosh, C.J. Oocyte expression, secretion and somatic cell interaction of mouse bone morphogenetic protein 15 during the peri-ovulatory period. Reprod. Fertil. Dev. 2015, 27, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.Q.; Ou, S.B.; Zhang, N.F.; Ren, L.; Wei, L.N.; Zhang, Q.X.; Yang, D.Z. Increased GDF9 and BMP15 mRNA levels in cumulus granulosa cells correlate with oocyte maturation, fertilization, and embryo quality in humans. Reprod. Biol. Endocrin 2014, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.K.; Otsuka, F.; Shimasaki, S. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J. Biol. Chem. 2003, 278, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Jeon, S.; Jeong, J.H.; Park, M.; Lee, D.R.; Yoon, T.K.; Choi, D.H.; Choi, Y. Identification and Characterization of LHX8 DNA Binding Elements. Balsaenggwa Saengsig 2012, 16, 379–384. [Google Scholar] [PubMed]
- Abir, R.; Fisch, B.; Johnson, M.H. BMP15, fertility and the ovary. Reprod. Biomed. Online 2014, 29, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Bayne, R.A.L.; Kinnell, H.L.; Coutts, S.M.; He, J.; Childs, A.J.; Anderson, R.A. GDF9 is transiently expressed in oocytes before follicle formation in the human fetal ovary and is regulated by a novel NOBOX transcript. PLoS ONE 2015, 10, e0119819. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Suzuki, H.; Jagarlamudi, K.; Golnoski, K.; McGuire, M.; Lopes, R.; Pachnis, V.; Rajkovic, A. LHX8 regulates primordial follicle activation and postnatal folliculogenesis. BMC Biol. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.J.; Reader, K.L.; Lun, S.; Western, A.; Lawrence, S.; McNatty, K.P.; Juengel, J.L. The cooperative effect of growth and differentiation factor-9 and bone morphogenetic protein (BMP)-15 on granulosa cell function is modulated primarily through BMP receptor II. Endocrinology 2008, 149, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Jaatinen, R.; Laitinen, M.P.; Vuojolainen, K.; Aaltonen, J.; Louhio, H.; Heikinheimo, K.; Lehtonen, E.; Ritvos, O. Localization of growth differentiation factor-9 (GDF-9) mRNA and protein in rat ovaries and cDNA cloning of rat GDF-9 and its novel homolog GDF-9B. Mol. Cell. Endocrinol. 1999, 156, 189–193. [Google Scholar] [CrossRef]
- Gene Regulation–Programs. Available online: www.gene-regulation.com/pub/programs.html (accessed on 2 July 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Q.; Wang, Y.; Wang, H. Identification and Analysis of Regulatory Elements in Porcine Bone Morphogenetic Protein 15 Gene Promoter. Int. J. Mol. Sci. 2015, 16, 25759-25772. https://doi.org/10.3390/ijms161025759
Wan Q, Wang Y, Wang H. Identification and Analysis of Regulatory Elements in Porcine Bone Morphogenetic Protein 15 Gene Promoter. International Journal of Molecular Sciences. 2015; 16(10):25759-25772. https://doi.org/10.3390/ijms161025759
Chicago/Turabian StyleWan, Qianhui, Yaxian Wang, and Huayan Wang. 2015. "Identification and Analysis of Regulatory Elements in Porcine Bone Morphogenetic Protein 15 Gene Promoter" International Journal of Molecular Sciences 16, no. 10: 25759-25772. https://doi.org/10.3390/ijms161025759
APA StyleWan, Q., Wang, Y., & Wang, H. (2015). Identification and Analysis of Regulatory Elements in Porcine Bone Morphogenetic Protein 15 Gene Promoter. International Journal of Molecular Sciences, 16(10), 25759-25772. https://doi.org/10.3390/ijms161025759