Discovery of a Potent Anti-Yeast Triterpenoid Saponin, Clematoside-S from Urena lobata L.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of Compounds 1–2 from the Leaves of Urena lobata


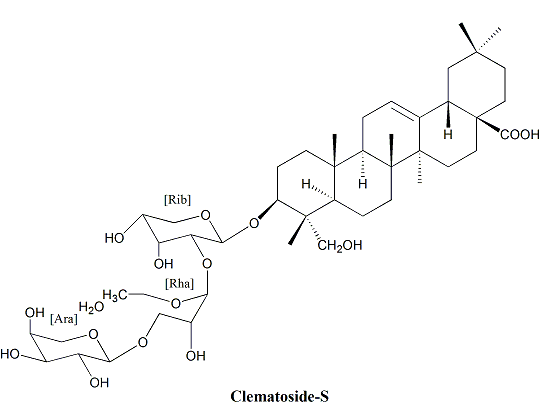
2.2. Identification of Isolated Compounds 1–2
| Position | δC | δH (Mult., J in Hz) | HMBC |
|---|---|---|---|
| 1 | 39.67 | 0.98 (overlap), 1.60 (m) | - |
| 2 | 26.58 | 1.74 (m), 1.85 (m) | - |
| 3 | 82.28 | 3.61 (m) | C-4, C-23, C-1' |
| 4 | 43.95 | - | - |
| 5 | 48.15 | 1.26 (m) | C-4, C-10, C-23, C-25 |
| 6 | 18.80 | 1.36 (m), 1.51 (m) | - |
| 7 | 33.39 | 1.26 (m), 1.63 (m) | - |
| 8 | 40.50 | - | - |
| 9 | 49.00 | 1.62 (m) | C-5, C-8, C-10, C-11 |
| 10 | 37.61 | - | |
| 11 | 24.52 | 1.87 (m), 1.89 (m) | C-12, C-13 |
| 12 | 123.60 | 5.23 (m) | - |
| 13 | 145.21 | - | - |
| 14 | 42.96 | - | - |
| 15 | 28.82 | 1.07 (m), 1.76 (m) | - |
| 16 | 20.05 | 1.61 (m), 2.01 (m) | C-28 |
| 17 | 47.63 | - | - |
| 18 | 42.72 | 2.84 (m) | C-12, C-13, C-17, C-28 |
| 19 | 47.22 | 1.12 (m), 1.69 (m) | - |
| 20 | 31.59 | - | - |
| 21 | 34.88 | 1.20 (m), 1.39 (m) | - |
| 22 | 33.81 | 1.53 (m), 1.74 (m) | - |
| 23 | 13.79 | 0.70 (s) | C-3, C-4, C-5, C-24 |
| 24 | 43.95 | 3.37 (m), 3.53 (m) | C-4, C-23 |
| 25 | 16.40 | 0.96 (s) | C-1, C-9, C-10 |
| 26 | 17.75 | 0.80 (s) | C-7, C-8, C-9, C-14 |
| 27 | 26.46 | 1.17 (s) | C-13, C-14, C-15 |
| 28 | 181.88 | - | - |
| 29 | 33.57 | 0.90 (s) | C-19, C-20, C-21, C-30 |
| 30 | 23.97 | 0.93 (s) | C-19, C-20, C-21, C-29 |
| 1' | 104.63 | 4.51 (d, J = 6.0) | C-3, C-5' |
| 2' | 76.33 | 3.69 (m) | C-1', C-3' |
| 3' | 74.21 | 3.69 (m) | C-1', C-2' |
| 4' | 69.64 | 3.75 (dd, J = 13.8, 6.6) | - |
| 5' | 65.44 | 3.5 (d, J =6.6), 3.83 (d, J = 6.6) | C-1', C-3' |
| 1'' | 101.52 | 5.21 (d, J = 1.6) | C-2', C-2'', C-3'', C-5'' |
| 2'' | 71.73 | 4.05 (d, J = 2.4) | C-3'', C-4'' |
| 3'' | 80.70 | 3.83 (d, J = 13.8) | C-4'', C-1''' |
| 4'' | 72.96 | 3.83 (d, J = 13.8) | C-3'', C-5'' |
| 5'' | 70.33 | 3.90 (d, J = 7.2) | - |
| 6'' | 17.99 | 1.23 (m) | C-5'' |
| 1''' | 104.18 | 4.99 (d, J = 4.3) | C-3'', C-3''' |
| 2''' | 72.60 | 3.67 (d, J = 3.2) | C-1''', C-3''' |
| 3''' | 68.66 | 3.96 (d, J = 3.2) | C-1''' |
| 4''' | 70.17 | 3.76 (dd, J = 13.2, 6.6) | C-1''', C-4''' |
| 5''' | 65.13 | 3.67 (d, J = 13.2), 3.89 (d, J = 13.2) | - |
2.3. Antimicrobial Activity of Isolated Active Compound
| Microorganisms Strain | Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|---|
| Extract a | Fra. 4 b | 1 c | 2 c | Negative Control d | Positive Control e | |
| Gram negative bacteria | ||||||
| Eschericha coli ATCC 25922 | 0 | NT f | 0 | 0 | 0 | 35 |
| Salmonella typhimurium ATCC 14028 | 0 | NT | 0 | 0 | 0 | 28 |
| Gram positive bacteria | ||||||
| Staphylpcocuus aureus ATCC 25923 | 0 | NT | 0 | 0 | 0 | 28 |
| Bacillus subtilis ATCC 21216 | 0 | NT | 0 | 0 | 0 | 18 |
| Bacillus cereus ATCC 10231 | 0 | NT | 0 | 0 | 0 | 15 |
| Bacillus laterosporus ATCC 64 | 0 | NT | 0 | 0 | 0 | 21 |
| Fungi | ||||||
| Aspergillus flavus ATCC 204304 | 0 | NT | 0 | 0 | 0 | 25 |
| Aspergillus niger ATCC 16404 | 9 | NT | 0 | 9 | 0 | 20 |
| Rhizopus oryzae ATCC 9363 | 0 | NT | 0 | 0 | 0 | 28 |
| Pencicillum citrinum ATCC 14994 | 0 | NT | 0 | 0 | 0 | 26 |
| Yeasts g | ||||||
| Candida albicans ATCC 50013 | 0 | NT | 0 | 0 | 0 | 0 |
| Saccharomyces cerevisiae ATCC 204508 | 15 | 16 | 0 | 20 | 0 | 21 |
| Saccharomyces cerevisiae AY529515.1 | 17 | NT | 0 | 11 | 0 | 21 |
| Saccharomyces cerevisiae AJ746340.1 | 14 | NT | 0 | 12 | 0 | 21 |
| Saccharomyces cerevisiae JX103178.1 | 14 | NT | 0 | 12 | 0 | 21 |
| Saccharomyces boulardii KG254081.1 | 17 | NT | 0 | 13 | 0 | 21 |
| Yest Strains | MIC (μg/mL) | MBC (μg/mL) |
|---|---|---|
| Saccharomyces cerevisiae ATCC 204505 | 0.61 | 2.42 |
| Saccharomyces cerevisiae\AY529515.1 | 1.21 | 4.84 |
| Saccharomyces cerevisiae\AJ746340.1 | 9.80 | 9.80 |
| Saccharomyces cerevisiae\JX103178.1 | 1.21 | 4.84 |
| Saccharomyces boulardii\KG254081.1 | 2.42 | 9.80 |
3. Experimental
3.1. General Procedure
3.2. Plant Material and Regents
3.3. Extraction and Isolation of the Active Compound
3.4. Acid Hydrolysis and GC-MS of Sugars
3.5. Antimicrobial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goretti, M.; Turchetti, B.; Buratta, M.; Branda, E.; Cxorazzi, L.; Vaughan-Martini, A.; Buzzini, P. In vitro antimycotic activity of a Williopsis saturnus killer protein against food spoilage yeasts. Int. J. Food Microbiol. 2009, 131, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Belletti, N.; Kamdem, S.S.; Patrignani, F.; Lanciotti, R.; Covelli, A.; Gardini, F. Antimicrobial activity of aroma compounds against Saccharomyces cerevisiae and improvement of microbiological stability of soft drinks as assessed by logistic regression. Appl. Environ. Microbiol. 2007, 73, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, V.; Querol, A. The prevalence and control of spoilage yeasts in foods and beverages. Trends Food Sci. Technol. 1999, 10, 156–165. [Google Scholar] [CrossRef]
- Tserennadmid, R.; Takó, M.; Galgóczy, L.; Papp, T.; Pesti, M.; Vágvölgyi, C.; Almássy, K.; Krisch, J. Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int. J. Food Microbiol. 2011, 144, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Gottardi, D.; Malik, A.; Guerzoni, M.E. Anti-yeast activity of mentha oil and vapours through in vitro and in vivo (real fruit juices) assays. Food Chem. 2013, 137, 108–114. [Google Scholar] [CrossRef] [PubMed]
- De las Heras, B.; Slowing, K.; Benedí, J.; Carretero, E.; Ortega, T.; Toledo, C.; Bermejo, P.; Iglesias, I.; Abad, M.J.; Gómez-Serranillos, P.; et al. Antiinflammatory and antioxidant activity of plants used in traditional medicine in Ecuador. J. Ethnopharmacol. 1998, 61, 161–166. [Google Scholar]
- Pieme, C.A.; Penlap, V.N.; Ngogang, J.; Costache, M. In vitro cytotoxicity and antioxidant activities of five medicinal plants of Malvaceae family from Cameroon. Environ. Toxicol. Pharmacol. 2010, 29, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, U.K.; Gupta, M.; Manikandan, L.; Bhattacharya, S. Antibacterial activity of Urena lobataroot. Fitoterapia 2001, 72, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, P.A.; Capriles, V.A. Antibacterial properties of tropical plants from Puerto Rico. Phytomedicine 2006, 13, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zeng, H.S.; Liu, C.; Chen, Y.; Zhong, M.Y. Simultaneous quantification of protocatechuic acid, caffeic acid, quercetin and kaempferol in Urena lobata L. by HPLC-VWD. Lat. Am. J. Pharm. 2013, 32, 272–276. [Google Scholar]
- Morelli, G.F.; Cairoli, P.; Speranza, G.; Alamgir, M.; Rajia, S. Triglycerides from Urena lobata. Fitoterapia 2006, 77, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Bi, Y.F.; Jing, L.L.; Zhou, S.A.; Kong, D.Y. Two new compounds from Urena lobata L. J. Asian Nat. Prod. Res. 2010, 12, 962–967. [Google Scholar] [CrossRef] [PubMed]
- John, L.M.D.; Tinto, W.F. Revised 13C-NMR assignments for the biologically active butyrolactone (−)-trachelogenin. J. Nat. Prod. 1992, 55, 1313–1314. [Google Scholar] [CrossRef]
- Lima, O.O.A.; Braz-Filho, R. Dibenzylbutyrolactone lignans and coumarins from Ipomoea cairica. J. Braz. Chem. Soc. 1997, 8, 235–238. [Google Scholar] [CrossRef]
- Boldizsár, I.; Kraszni, M.; Tóth, F.; Noszál, B.; Molnár-Perl, I. Complementary fragmentation pattern analysis by gas chromatography–mass spectrometry and liquid chromatography tandem mass spectrometry confirmed the precious lignan content of Cirsium weeds. J. Chromatogr. A 2010, 1133, 6281–6289. [Google Scholar] [CrossRef]
- Yokosuka, A.; Sano, T.; Hashimoto, K.; Sakagami, H.; Mimaki, Y. Triterpene glycosides from the whole plant of Anemone hupehensis var. japonica and their cytotoxic activity. Chem. Pharm. Bull. 2009, 57, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Ohtani, K.; Wei, J.X.; Tanaka, O. Saponins from Anemone rivularis. Planta Med. 1984, 50, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Sati, O.P.; Uniyal, S.K.; Bahuguna, S.; Kikuchi, T. Clematoside-S, a triterpenoid saponin from the roots of Clematis grata. Phytochemistry 1990, 29, 3676–3678. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Okabe, H.; Mihashi, K. Gas-liquid chromatographic separation of aldose enantiomers as trimethylsilyl ethers of methyl 2-(polyhydroxyakyl)-thiazolidine-4-(R)-carboxylates. Chem. Pharm. Bull. 1987, 35, 501–506. [Google Scholar] [CrossRef]
- Mitaine-Offer, A.C.; Pénez, N.; Miyamoto, T.; Delaude, C.; Mirjolet, J.F.; Duchamp, O.; Lacaille-Dubois, M.A. Acylated triterpene saponins from the roots of Securidaca longepedunculata. Phytochemistry 2010, 71, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, S.; Jedrejek, D.; Kowalczyk, M.; Pecio, L.; Masullo, M.; Piacente, S.; Macias, F.A.; Simonet, A.M.; Oleszek, W.; Stochmal, A. Triterpene saponins from the aerial parts of Trifolium medium L. var. sarosiense. J. Agric. Food Chem. 2013, 61, 9789–9796. [Google Scholar] [CrossRef]
- Yan, L.H.; Xu, L.Z.; Lin, L.; Yang, S.L.; Feng, Y.L. Triterpenoid saponins from the stems of Clematis parviloba. J. Asian Nat. Prod. Res. 2009, 11, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.C.; Jia, L.R.; Zhang, Y.; Cen, J.Q.; Chen, X.; Gao, H.; Huang, Y.N. Antibrowning and antimicrobial activities of the water-soluble extract from pine needles of Cedrus deodara. J. Food Sci. 2011, 76, 318–323. [Google Scholar] [CrossRef]
- Zeng, W.C.; Zhu, R.X.; Jia, L.R.; Gao, H.; Zheng, Y.; Sun, Q. Chemical composition, antimicrobial and antioxidant activities of essential oil from Gnaphlium affine. Food Chem. Toxicol. 2011, 49, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.-L.; Liao, Y.; Wang, J.; Liu, X.-Y.; Zhong, K.; Huang, Y.-N.; Gao, H.; Gao, B.; Xu, Z.-J. Discovery of a Potent Anti-Yeast Triterpenoid Saponin, Clematoside-S from Urena lobata L. Int. J. Mol. Sci. 2015, 16, 4731-4743. https://doi.org/10.3390/ijms16034731
Gao X-L, Liao Y, Wang J, Liu X-Y, Zhong K, Huang Y-N, Gao H, Gao B, Xu Z-J. Discovery of a Potent Anti-Yeast Triterpenoid Saponin, Clematoside-S from Urena lobata L. International Journal of Molecular Sciences. 2015; 16(3):4731-4743. https://doi.org/10.3390/ijms16034731
Chicago/Turabian StyleGao, Xiao-Ling, Ying Liao, Jie Wang, Xiao-Yan Liu, Kai Zhong, Yi-Na Huang, Hong Gao, Bo Gao, and Zheng-Jun Xu. 2015. "Discovery of a Potent Anti-Yeast Triterpenoid Saponin, Clematoside-S from Urena lobata L." International Journal of Molecular Sciences 16, no. 3: 4731-4743. https://doi.org/10.3390/ijms16034731