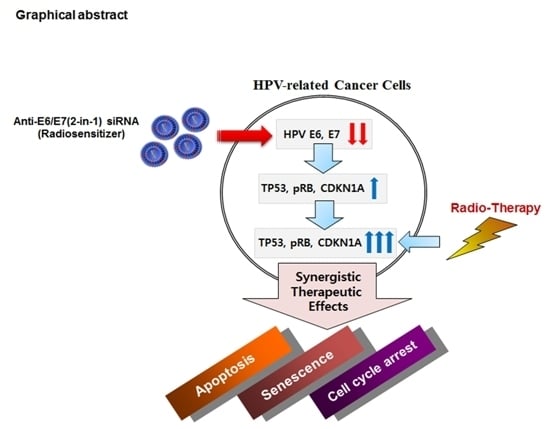
Human Papillomavirus E6/E7-Specific siRNA Potentiates the Effect of Radiotherapy for Cervical Cancer in Vitro and in Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of HPV18 E6/E7-Specific Lead siRNAs in Combination with Radiation on Cervical Cancer Cells

| 426 siRNA (nM) | Radiation (Gy) | Cytotoxicity (%) | CI * |
|---|---|---|---|
| HPV18-Type HeLa Cells | |||
| 5 | 4.8 | 86 | 0.73 |
| 25 | 4.8 | 83 | 0.86 |
| 5 | 2 | 43 | 0.9 |
| 25 | 2 | 51 | 0.9 |
| 497 siRNA (nM) | Radiation (Gy) | Cytotoxicity (%) | CI * |
| HPV16-Type SiHa Cells | |||
| 50 | 2 | 78 | 0.63 |
| 25 | 2 | 68 | 0.71 |
| 50 | 4 | 77 | 0.80 |
2.2. In Vivo Effects of HPV18 E6/E7-Specific siRNA in Combination with Radiation


2.3. The Effect of Chemically-Modified E6/E7-Specific siRNAs on Silencing Efficiency and Serum Stability
| Name | Derivative Number | Sequence | Selected Candidates | |
|---|---|---|---|---|
| HPV18-type siRNA 426 (19mer) | 1 | Sense (S) | 5ʹ-caaccgagcacgacaggaa dTdT-3ʹ | |
| Antisense (AS) | 5ʹ-uuccugucgugcucgguug dTdT-3ʹ | |||
| 2 | 6FC (S) | 5ʹ- caaccgagcacgacaggaa dTdT-3ʹ | ||
| Antisense (AS) | 5ʹ-uuccugucgugcucgguug dTdT-3ʹ | |||
| 3 | 6FC (S) | 5ʹ- caaccgagcacgacaggaa dTdT-3ʹ | ||
| 4MeU (AS) | 5ʹ-uucc ugucgugcucgguug dTdT-3ʹ | |||
| 4 | 6FC (S) | 5ʹ- caaccgagcacgacaggaa dTdT-3ʹ | ||
| 6MeU (AS) | 5ʹ- uuccugucgugcucgguug dTdT-3ʹ | |||
| 5 | 5MeG (S) | 5ʹ-caacc gagcacgacaggaa dTdT-3ʹ | d5 | |
| Antisense (AS) | 5ʹ-uuccugucgugcucgguug dTdT-3ʹ | |||
| 6 | 5MeG (S) | 5ʹ-caacc gagcacgacaggaa dTdT-3ʹ | ||
| 4MeU (AS) | 5ʹ-uucc ugucgugcucgguug dTdT-3ʹ | |||
| 7 | 5MeG (S) | 5ʹ-caacc gagcacgacaggaa dTdT-3ʹ | ||
| 6MeU (AS) | 5ʹ- uuccugucgugcucgguug dTdT-3ʹ | |||
| HPV16-type siRNA 497 (21mer) | 1 | Sense (S) | 5ʹ-gaccggucgauguaugucuug-3ʹ | |
| Antisense (AS) | 5ʹ-agacauacaucgaccggucca-3ʹ | |||
| 2 | Sense (S) | 5ʹ-gaccggucgauguaugucuug-3ʹ | d2 | |
| 3MeU (AS) | 5ʹ-agaca uacaucgaccggucca-3ʹ | |||
| 3 | 6MeU (S) | 5ʹ-gaccgg ucgauguaugucuug-3ʹ | ||
| Antisense (AS) | 5ʹ-agacauacaucgaccggucca-3ʹ | |||
| 4 | 6MeU (S) | 5ʹ-gaccgg ucgauguaugucuug-3ʹ | ||
| 3MeU (AS) | 5ʹ-agaca uacaucgaccggucca-3ʹ | |||

2.4. Synergistic Effect of E6/E7-Specific siRNA Pool in Combination with Radiation on Cervical Cancer Cells

3. Experimental Section
3.1. Cell Lines and Western Blotting
3.2. In Vitro Models of siRNA Transfection and γ-Irradiation
3.3. Flow Cytometric Analysis and Senescence-Associated β-Galactosidase Assay
3.4. Quantitative Real-Time PCR Analysis
3.5. Serum Stability
3.6. In Vivo Xenografts, Imaging and Immunohistochemical Analysis
3.7. Chou and Talalay Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huibregtse, J.M.; Scheffner, M.; Howley, P.M. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol. Cell. Biol. 1993, 13, 775–784. [Google Scholar] [PubMed]
- Zur Hausen, H.; de Villiers, E.M. Human papillomaviruses. Annu. Rev. Microbiol. 1994, 48, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Woodman, C.B.; Collins, S.I.; Young, L.S. The natural history of cervical HPV infection: Unresolved issues. Nat. Rev. Cancer 2007, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hainaut, P.; Hernandez, T.; Robinson, A.; Rodriguez-Tome, P.; Flores, T.; Hollstein, M.; Harris, C.C.; Montesano, R. Iarc database of p53 gene mutations in human tumors and cell lines: Updated compilation, revised formats and new visualisation tools. Nucleic Acids Res. 1998, 26, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Munger, K.; Byrne, J.C.; Howley, P.M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc. Natl. Acad. Sci. USA 1991, 88, 5523–5527. [Google Scholar] [CrossRef] [PubMed]
- Dyson, N.; Howley, P.M.; Munger, K.; Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989, 243, 934–937. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, D.; Petti, L.M. The E5 proteins. Virology 2013, 445, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Maufort, J.P.; Shai, A.; Pitot, H.C.; Lambert, P.F. A role for HPV16 E5 in cervical carcinogenesis. Cancer Res. 2010, 70, 2924–2931. [Google Scholar] [CrossRef] [PubMed]
- Haasnoot, J.; Westerhout, E.M.; Berkhout, B. RNA interference against viruses: Strike and counterstrike. Nat. Biotechnol. 2007, 25, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Milner, J. Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene 2002, 21, 6041–6048. [Google Scholar] [CrossRef] [PubMed]
- Butz, K.; Ristriani, T.; Hengstermann, A.; Denk, C.; Scheffner, M.; Hoppe-Seyler, F. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene 2003, 22, 5938–5945. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.H.; Alexander, K.A. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J. Virol. 2003, 77, 6066–6069. [Google Scholar] [CrossRef] [PubMed]
- Yoshinouchi, M.; Yamada, T.; Kizaki, M.; Fen, J.; Koseki, T.; Ikeda, Y.; Nishihara, T.; Yamato, K. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by E6 siRNA. Mol. Ther. 2003, 8, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Putral, L.; Hengst, K.; Minto, K.; Saunders, N.A.; Leggatt, G.; McMillan, N.A. Inhibition of cervical cancer cell growth in vitro and in vivo with lentiviral-vector delivered short hairpin RNA targeting human papillomavirus E6 and E7 oncogenes. Cancer Gene Ther. 2006, 13, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Sima, N.; Wang, W.; Kong, D.; Deng, D.; Xu, Q.; Zhou, J.; Xu, G.; Meng, L.; Lu, Y.; Wang, S.; et al. RNA interference against HPV16 E7 oncogene leads to viral E6 and E7 suppression in cervical cancer cells and apoptosis via upregulation of Rb and p53. Apoptosis 2008, 13, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Yamato, K.; Yamada, T.; Kizaki, M.; Ui-Tei, K.; Natori, Y.; Fujino, M.; Nishihara, T.; Ikeda, Y.; Nasu, Y.; Saigo, K.; et al. New highly potent and specific E6 and E7 siRNAs for treatment of HPV16 positive cervical cancer. Cancer Gene Ther. 2008, 15, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Erkin, O.C.; Kwon, M.J.; Kim, S.H.; Jung, J.I.; Oh, Y.K.; Her, S.W.; Ju, W.; Choi, Y.L.; Song, S.Y.; et al. The synergistic therapeutic effect of cisplatin with human papillomavirus E6/E7 short interfering RNA on cervical cancer cell lines in vitro and in vivo. Int. J. Cancer 2012, 130, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, S.Y.; Chen, T.T.; Chen, J.C.; Chiou, C.L.; Huang, T.M. Cisplatin restores p53 function and enhances the radiosensitivity in HPV16 E6 containing SiHa cells. J. Cell. Biochem. 2004, 91, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.J.; Park, I.C.; Park, M.J.; Woo, S.H.; Hong, S.I.; Chung, H.Y.; Kim, T.H.; Lee, Y.S.; Rhee, C.H.; Lee, S.J. Enhancement of radiation response in human cervical cancer cells in vitro and in vivo by arsenic trioxide (As2O3). FEBS Lett. 2002, 519, 195–200. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Shibata, K.; Kajiyama, H.; Terauchi, M.; Nawa, A.; Kikkawa, F. Aminopeptidase N (APV)/CD13 inhibitor, Ubenimex, enhances radiation sensitivity in human cervical cancer. BMC Cancer 2008, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L., III; Walker, J.L.; Gersell, D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N. Engl. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Peters, W.A., III; Liu, P.Y.; Barrett, R.J., 2nd; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.; Shu, Y.; Haque, F.; Abdelmawla, S.; Guo, P. Thermodynamically stable RNA three-way junction for constructing multifunctional nanoparticles for delivery of therapeutics. Nat. Nanotechnol. 2011, 6, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Pi, F.; Sharma, A.; Rajabi, M.; Haque, F.; Shu, D.; Leggas, M.; Evers, B.M.; Guo, P. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Baumer, S.; Baumer, N.; Appel, N.; Terheyden, L.; Fremerey, J.; Schelhaas, S.; Wardelmann, E.; Buchholz, F.; Berdel, W.E.; Muller-Tidow, C. Antibody-mediated delivery of anti-KRAS-siRNA in vivo overcomes therapy resistance in colon cancer. Clin. Cancer Res. 2015, 21, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Rychahou, P.; Haque, F.; Shu, Y.; Zaytseva, Y.; Weiss, H.L.; Lee, E.Y.; Mustain, W.; Valentino, J.; Guo, P.; Evers, B.M. Delivery of RNA nanoparticles into colorectal cancer metastases following systemic administration. ACS Nano 2015, 9, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, F.; Fechtner, M.; Dames, S.; Aygun, H.; Klippel, A.; Pronk, G.J.; Giese, K.; Kaufmann, J. Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 2003, 31, 2705–2716. [Google Scholar] [CrossRef] [PubMed]
- Allerson, C.R.; Sioufi, N.; Jarres, R.; Prakash, T.P.; Naik, N.; Berdeja, A.; Wanders, L.; Griffey, R.H.; Swayze, E.E.; Bhat, B. Fully 2ʹ-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J. Med. Chem. 2005, 48, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Behlke, M.A. Chemical modification of siRNAs for in vivo use. Oligonucleotides 2008, 18, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Yamato, K.; Egawa, N.; Endo, S.; Ui-Tei, K.; Yamada, T.; Saigo, K.; Hyodo, I.; Kiyono, T.; Nakagawa, I. Enhanced specificity of HPV16 E6E7 siRNA by RNA-DNA chimera modification. Cancer Gene Ther. 2011, 18, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.A.; Blessing, J.A.; Vaccarello, L.; Roman, L.D.; Gynecologic Oncology Group, S. Phase II clinical trial of docetaxel in refractory squamous cell carcinoma of the cervix: A gynecologic oncology group study. Am. J. Clin. Oncol. 2007, 30, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Long, H.J., III; Bundy, B.N.; Grendys, E.C., Jr.; Benda, J.A.; McMeekin, D.S.; Sorosky, J.; Miller, D.S.; Eaton, L.A.; Fiorica, J.V. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: A gynecologic oncology group study. J. Clin. Oncol. 2005, 23, 4626–4633. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Yakushiji, M.; Noda, K.; Ikeda, M.; Kudoh, R.; Yajima, A.; Tomoda, Y.; Terashima, Y.; Takeuchi, S.; Hiura, M.; et al. Phase II study of irinotecan and cisplatin as first-line chemotherapy in advanced or recurrent cervical cancer. Oncology 2000, 58, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Pectasides, D.; Fountzilas, G.; Papaxoinis, G.; Pectasides, E.; Xiros, N.; Sykiotis, C.; Koumarianou, A.; Psyrri, A.; Panayiotides, J.; Economopoulos, T. Carboplatin and paclitaxel in metastatic or recurrent cervical cancer. Int. J. Gynecolo. Cancer 2009, 19, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Choung, S.; Kim, Y.J.; Kim, S.; Park, H.O.; Choi, Y.C. Chemical modification of siRNA to improve serum stability without loss of efficacy. Biochem. Biophys. Res. Commun. 2006, 342, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Hansson, B.; Norman, I.; Gaberi, V.; Mints, M.; Hjerpe, A.; Karlsen, F.; Johansson, B. Expression of E6/E7 mRNA from “high risk” human papillomavirus in relation to cin grade, viral load and p16ink4a. Int. J. Oncol. 2006, 29, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Fujii, T.; Saito, M.; Nindl, I.; Ono, A.; Kubushiro, K.; Tsukazaki, K.; Mukai, M.; Nozawa, S. Overexpression of p16 ink4a as an indicator for human papillomavirus oncogenic activity in cervical squamous neoplasia. Int. J. Gynecol. Cancer 2006, 16, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.P.; Stemmer-Rachamimov, A.O.; Shaw, J.; Roy, J.E.; Koh, J.; Louis, D.N. Immunohistochemical survey of p16ink4a expression in normal human adult and infant tissues. Lab. Investig. 1999, 79, 1137–1143. [Google Scholar] [PubMed]
- Klaes, R.; Friedrich, T.; Spitkovsky, D.; Ridder, R.; Rudy, W.; Petry, U.; Dallenbach-Hellweg, G.; Schmidt, D.; von Knebel Doeberitz, M. Overexpression of p16(ink4a) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int. J. Cancer 2001, 92, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.M.; Ho, T.H.; Hui, K.M. P16ink4a-silencing augments DNA damage-induced apoptosis in cervical cancer cells. Oncogene 2007, 26, 6050–6060. [Google Scholar] [CrossRef] [PubMed]
- Van de Putte, G.; Holm, R.; Lie, A.K.; Trope, C.G.; Kristensen, G.B. Expression of p27, p21, and p16 protein in early squamous cervical cancer and its relation to prognosis. Gynecol. Oncol. 2003, 89, 140–147. [Google Scholar] [CrossRef]
- Munger, K.; Basile, J.R.; Duensing, S.; Eichten, A.; Gonzalez, S.L.; Grace, M.; Zacny, V.L. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 2001, 20, 7888–7898. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, F.; Banks, L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 2001, 20, 7874–7887. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.S.; Rajasekaran, N.; Song, S.Y.; Kim, Y.D.; Hong, S.; Choi, H.J.; Kim, Y.S.; Choi, J.-S.; Choi, Y.-L.; Shin, Y.K. Human Papillomavirus E6/E7-Specific siRNA Potentiates the Effect of Radiotherapy for Cervical Cancer in Vitro and in Vivo. Int. J. Mol. Sci. 2015, 16, 12243-12260. https://doi.org/10.3390/ijms160612243
Jung HS, Rajasekaran N, Song SY, Kim YD, Hong S, Choi HJ, Kim YS, Choi J-S, Choi Y-L, Shin YK. Human Papillomavirus E6/E7-Specific siRNA Potentiates the Effect of Radiotherapy for Cervical Cancer in Vitro and in Vivo. International Journal of Molecular Sciences. 2015; 16(6):12243-12260. https://doi.org/10.3390/ijms160612243
Chicago/Turabian StyleJung, Hun Soon, Nirmal Rajasekaran, Sang Yong Song, Young Deug Kim, Sungyoul Hong, Hyuck Jae Choi, Young Seok Kim, Jong-Sun Choi, Yoon-La Choi, and Young Kee Shin. 2015. "Human Papillomavirus E6/E7-Specific siRNA Potentiates the Effect of Radiotherapy for Cervical Cancer in Vitro and in Vivo" International Journal of Molecular Sciences 16, no. 6: 12243-12260. https://doi.org/10.3390/ijms160612243