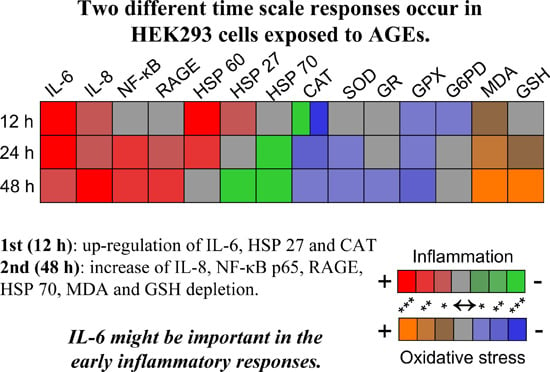
AGEs-Induced IL-6 Synthesis Precedes RAGE Up-Regulation in HEK 293 Cells: An Alternative Inflammatory Mechanism?
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Degree of BSA Glycation
2.2. Cell Viability
2.3. Inflammatory Response Evoked in HEK 293 Cells
| Cytokine pg/mL | Treatment | Cell Lysate | Cell Culture Medium | |||||
|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | |||
| IL-2 | 200 μg/mL | BSA | n.d. | n.d. | 0.54 ± 0.05 | n.d. | n.d. | n.d. |
| AGEs | n.d. | n.d. | 1.27 ± 0.15 | n.d. | n.d. | n.d. | ||
| Fold change | − | − | 2.34 ** | − | − | − | ||
| IL-4 | 200 μg/mL | BSA | n.d. | n.d. | 0.3 ± 0.08 | n.d. | n.d. | n.d. |
| AGEs | n.d. | n.d. | 0.74 ± 0.06 | n.d. | n.d. | n.d. | ||
| Fold change | − | − | 2.48 *** | − | − | − | ||
| IL-6 | 200 μg/mL | BSA | 0.72 ± 0.12 | 0.42 ± 0.12 | 0.26 ± 0.08 | 7.67 ± 0.53 | 5.45 ± 0.22 | 4.65 ± 0.13 |
| AGEs | 2.37 ± 0.29 | 0.37 ± 0.13 | 0.23 ± 0.01 | 79.49 ± 5.01 | 16.41 ± 2.3 | 11.61 ± 1.54 | ||
| Fold change | 3.31 *** | 0.89 | 0.88 | 10.37 *** | 3.01 *** | 2.5 ** | ||
| IL-8 | 200 μg/mL | BSA | 10.13 ± 2.4 | 11.47 ± 1.44 | 33.9 ± 2.34 | 54.4 ± 4.21 | 75.82 ± 6.16 | 85.82 ± 7.2 |
| AGEs | 11.29 ± 3.76 | 20.02 ± 3.45 | 65.69 ±5.98 | 74.4 ± 4.03 | 111.69 ± 9.21 | 237.6 ± 12.4 | ||
| Fold change | 1.11 | 1.75 *** | 1.94 *** | 1.37 * | 1.47 * | 2.78 *** | ||
| IL-10 | 200 μg/mL | BSA | n.d. | n.d. | 0.92 ± 0.13 | n.d. | n.d. | n.d. |
| AGEs | n.d. | n.d. | 2.43 ± 0.17 | n.d. | n.d. | n.d. | ||
| Fold change | − | − | 2.64 *** | − | − | − | ||
| GM-CSF | 200 μg/mL | BSA | 7.69 ± 1.49 | 8.34 ± 0.75 | 9.61 ± 0.24 | n.d. | n.d. | n.d. |
| AGEs | 9.72 ± 0.59 | 11.73 ± 0.75 | 18.33 ± 0.88 | n.d. | n.d. | n.d. | ||
| Fold change | 1.26 * | 1.41 *** | 1.91 *** | − | − | − | ||
| IFN-γ | 200 μg/mL | BSA | 14.68 ± 1.52 | 12.24 ± 0.98 | 12.6 ± 0.28 | n.d. | n.d. | n.d. |
| AGEs | 17.87 ± 1.11 | 22.08 ± 0.02 | 41.81 ± 3.5 | n.d. | n.d. | n.d. | ||
| Fold change | 1.22 ** | 1.8 *** | 3.32 *** | − | − | − | ||
| TNF-α | 200 μg/mL | BSA | n.d. | n.d. | 0.86 ± 0.03 | n.d. | n.d. | n.d. |
| AGEs | n.d. | n.d. | 2.84 ± 0.05 | n.d. | n.d. | n.d. | ||
| Fold change | − | − | 3.17 *** | − | − | − | ||

2.4. AGEs–BSA Exposure Activates NF-κB and RAGE Expression



2.5. HSPs Response to AGEs–BSA Exposure in HEK 293 Cells
2.6. Oxidative Stress Induced by AGEs–BSA Exposure and the Antioxidant Response of HEK 293 Cells
3. Experimental Section
3.1. AGEs–BSA Preparation and the Assessment of BSA Glycation
3.2. Cell Culture and Treatment
3.3. Preparation of Conditioned Media and Quantitation of Inflammatory Cytokines
3.4. Antioxidant Enzymes Activity
3.5. Oxidative Stress Markers
3.6. Preparation of Membrane Protein Fraction
3.7. Western Blotting
3.8. SOD Zymography
3.9. RNA Extraction and Semi-quantitative Real-Time PCR
| Gene | Accession Number | Oligonucleotide Sequence (5′–3′) | Ta (°C) | Amplicon Size (bp) |
|---|---|---|---|---|
| RAGE | NM_001136.4 | F: TGGATGAAGGATGGTGTG | 49 | 153 |
| R: GATGATGCTGATGCTGA | ||||
| HSP 27 | NM_001541.3 | F: GCCGACATTTGGACACAG | 58 | 184 |
| R: CTGCTACCTCTGGAGTGG | ||||
| HSP 60 | XM_005246518.1 | F: GTGGAAAAAGGAATCATTGACC | 58 | 83 |
| R: GTAGTTAACAGAGAGGCCACACC | ||||
| HSP 70 | XM_005272813.2 | F: AAGGAATTGGAGCAGATGTGTAAC | 58 | 244 |
| R: CAAGGGATGGTAACTTAG ATTCAGG | ||||
| GAPDH | NM_002046.4 | F: TGGTCTCCTCTGACTTCAAC | 58 | 222 |
| R: GTGAGGGTCTCTCTCTTCCT |
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Serban, A.I.; Costache, M.; Dinischiotu, A. Controversial behavior of aminoguanidine in the presence of either reducing sugars or soluble glycated bovine serum albumin. Carbohydr. Res. 2011, 346, 2872–2880. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Thorpe, S.R.; Baynes, J.W. Identification of carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J. Biol. Chem. 1986, 261, 8816–8821. [Google Scholar]
- Biemel, M.K.; Reihl, O.; Conrad, J.; Lederer, M.O. Formation pathways for lysine-arginine cross-links derived from hexoses and pentoses by Maillard processes. J. Biol. Chem. 2001, 276, 23405–23412. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Biemel, K.M.; Reihl, O.; Lederer, M.O.; Strauch, C.M.; Monnier, V.M. Glucosepane is a major protein cross-link of the senescent human extracellular matrix. Relationship with diabetes. J. Biol. Chem. 2005, 280, 12310–12315. [Google Scholar] [CrossRef] [PubMed]
- Serban, A.I.; Condac, E.; Costache, M.; Dinischiotu, A. The relationship between AGEs, Cu2+ and crosslinking of collagen. Rev. Roum. Chim. 2009, 54, 93–101. [Google Scholar]
- Bierhaus, A.; Nawroth, P.P. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 2009, 52, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Fritz, G. RAGE regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Rouhiainen, A.; Kuja-Panula, J.; Tumova, S.; Rauvala, H. RAGE-mediated cell signaling. Methods Mol. Biol. 2013, 963, 239–263. [Google Scholar] [PubMed]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Mechanisms of disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Rev. Endocrinol. 2008, 4, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.D. A race for RAGE ligands. Kidney Int. 2010, 78, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Fritz, G. RAGE: A single receptor fits multiple ligands. Trends Biochem. Sci. 2011, 36, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, P.; Chatzopoulou, M.; Pegklidou, K.; Demopoulos, V.J. RAGE: A multi-ligand receptor unveiling novel insights in health and disease. Curr. Med. Chem. 2010, 17, 2232–2252. [Google Scholar] [CrossRef] [PubMed]
- Serban, A.I.; Stanca, L.; Geicu, O.I.; Munteanu, M.C.; Costache, M.; Dinischiotu, A. Extracellular matrix is modulated in advanced glycation end products milieu via a RAGE receptor dependent pathway boosted by transforming growth factor-β1. J. Diabetes 2015, 7, 114–124. [Google Scholar] [CrossRef] [PubMed]
- San Martin, A.; Foncea, R.; Laurindo, F.R.; Ebensperger, R.; Griendling, K.K.; Leighton, F. Nox1-based NADPH oxidase-derived superoxide is required for VSMC activation by advanced glycation end-products. Free Radic. Biol. Med. 2007, 42, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Ricci, C.; Oddi, G.; Pesce, C.; Pugliese, F.; Block, K.; Abboud, H.E.; Giorgio, M.; Migliaccio, E.; et al. Ablation of the gene encoding p66Shc protects mice against AGE-induced glomerulopathy by preventing oxidant-dependent tissue injury and further AGE accumulation. Diabetologia 2007, 50, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, B.; Greensmith, L. Induction of heat shock proteins for protection against oxidative stress. Adv. Drug Deliv. Rev. 2009, 61, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Alford, K.A.; Glennie, S.; Turrell, B.R.; Rawlinson, L.; Saklatvala, J.; Dean, J.L. Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-β-activated kinase-1 (TAK1)-mediated signaling. J. Biol. Chem. 2007, 282, 6232–6241. [Google Scholar] [CrossRef] [PubMed]
- Borges, T.J.; Wieten, L.; van Herwijnen, M.J.; Broero, F.; van der Zee, R.; Bonorino, C.; van Eden, W. The anti-inflammatory mechanisms of Hsp70. Front. Immunol. 2012, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Simmons, N.L. A cultured human renal epithelioid cell line responsive to vasoactive intestinal peptide. Exp. Physiol. 1990, 75, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lenz, W.; Herten, M.; Gerzer, R.; Drummer, C. Regulation of natriuretic peptide (urodilatin) release in a human kidney cell line. Kidney Int. 1999, 55, 91–99. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, M.C.; Frasnelli, S.C.; Bastos, A.S.; Orrico, S.R.; Rossa, C.J. Modulation of cell proliferation, survival and gene expression by RAGE and TLR signaling in cells of the innate and adaptive immune response: Role of p38 MAPK and NF-KB. J. Appl. Oral Sci. 2014, 22, 185–193. [Google Scholar] [CrossRef]
- Von Laue, S.; Finidori, J.; Maamra, M.; Shen, X.Y.; Justice, S.; Dobson, P.R.; Ross, R.J. Stimulation of endogenous GH and interleukin-6 receptors selectively activates different Jaks and Stats, with a STAT5 specific synergistic effect of dexamethasone. J. Endocrinol. 2000, 165, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lafdil, F.; Kong, X.; Gao, B. Signal transducer and activator of transcription 3 in liver diseases: A novel therapeutic target. Int. J. Biol. Sci. 2011, 7, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.K.; Chui, R.K.; Tam, I.Y.; Lau, A.H.; Wong, Y.H. CCR1-mediated STAT3 tyrosine phosphorylation and CXCL8 expression in THP-1 macrophage-like cells involve pertussis toxin-insensitive Gα(14/16) signaling and IL-6 release. J. Immunol. 2012, 189, 5266–5276. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.W.; Ng, J.K.; Lee, M.M.; Chan, A.S.; Wong, Y.H. Interleukin-6 autocrine signaling mediates melatonin MT1/2 receptor-induced STAT3 Tyr705 phosphorylation. J. Pineal Res. 2012, 52, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Simhan, H.N.; Chura, J.C.; Rauk, P.N. The effect of the anti-inflammatory cytokines interleukin-4 and interleukin-10 on lipopolysaccharide-stimulated production of prostaglandin E2 by cultured human decidual cells. J. Reprod. Immunol. 2004, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Carstensen, M.; Ouwens, D.M. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Gong, H.; Chen, Z.; Liang, Y.; Yuan, J.; Zhang, G.; Wu, J.; Ye, Y.; Yang, C.; Nakai, A.; et al. Association of STAT3 with HSF1 plays a critical role in G-CSF-induced cardio-protection against ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2012, 52, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Sturrock, A.; Seedahmed, E.; Mir-Kasimov, M.; Boltax, J.; McManus, M.L.; Paine, R. GM-CSF provides autocrine protection for murine alveolar epithelial cells from oxidant-induced mitochondrial injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Kattla, J.J.; Carew, R.M.; Heljic, M.; Godson, C.; Brazil, D.P. Protein kinase B/Akt activity is involved in renal TGF-β1-driven epithelial-mesenchymal transition in vitro and in vivo. Am. J. Physiol. Renal Physiol. 2008, 295, F215–F225. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, S.; Roy, S.; Varma, S.; Bose, M. Pulmonary epithelial cells are a source of interferon-γ in response to Mycobacterium tuberculosis infection. Immunol. Cell Biol. 2007, 85, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Katsuoka, F.; Kawakami, Y.; Arai, T.; Imuta, H.; Fujiwara, M.; Kanma, H.; Yamashita, K. Type II alveolar epithelial cells in lung express receptor for advanced glycation end products (RAGE) gene. Biochem. Biophys. Res. Commun. 1997, 238, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, C.M.; Burkhart, M.A.; Mujtaba, M.G.; Subramaniam, P.S.; Johnson, H.M. The role of IFN-γ nuclear localization sequence in intracellular function. J. Cell Sci. 2003, 116, 3089–3098. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Cao, S.; Cui, J.Z.; Matsubara, J.A. Immuno-modulatory effect of IFN-γ in AMD and its role as a possible target for therapy. J. Clin. Exp. Ophthalmol. 2013, 2, 7. [Google Scholar]
- Bierhaus, A.; Schiekofer, S.; Schwaninger, M.; Andrassy, M.; Humpert, P.M.; Chen, J.; Hong, M.; Luther, T.; Henle, T.; Klöting, I.; et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes 2001, 50, 2792–2808. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Lazzerini, G.; del Turco, S.; Ratto, G.M.; Schmidt, A.M.; de Caterina, R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Choi, B.; Lee, K.; Lee, D.; Kang, D.; Lee, J.; Song, I.; Kim, H.; Lee, S.; Kim, H.; et al. Cytosolic HSP60 is involved in the NF-κB-dependent survival of cancer cells via IKK regulation. PLoS ONE 2010, 5, e9422. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Kudo, M.; Hagiwara, S.; Tabuchi, M.; Watanabe, T.; Munakata, H.; Sakurai, T. p38MAPK suppresses chronic pancreatitis by regulating HSP27 and BAD expression. Free Radic. Biol. Med. 2012, 52, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. P38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Giffard, R.G.; Han, R.Q.; Emery, J.F.; Duan, M.; Pittet, J.F. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology 2008, 109, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Nedić, O.; Rattan, S.I.; Grune, T.; Trougakos, I.P. Molecular effects of advanced glycation end products on cell signalling pathways, ageing andpathophysiology. Free Radic. Res. 2013, 47, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. AGE-receptor-1 counteracts cellular oxidant stress induced by AGEs via negative regulation of p66shc-dependent FKHRL1 phosphorylation. Am. J. Physiol. Cell Physiol. 2008, 294, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.; Khursigara, G.; Sun, X.; Franke, T.F.; Chao, M.V. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell Biol. 2001, 21, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Barkett, M.; Gilmore, T.D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 1999, 18, 6910–6924. [Google Scholar] [CrossRef] [PubMed]
- Lauder, A.; Castellanos, A.; Weston, K. c-Myb transcription is activated by protein kinase B (PKB) following interleukin 2 stimulation of T cells and is required for PKB-mediated protection from apoptosis. Mol. Cell Biol. 2001, 21, 5797–5805. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Zheng-Gang, L. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, B.; Mahimainathan, L.; Das, F.; Ghosh-Choudhury, N.; Ghosh Choudhury, G. Downregulation of catalase by reactive oxygen species via PI3 kinase/Akt signaling in mesangial cells. J. Cell. Physiol. 2007, 211, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Das, F.; Ghosh-Choudhury, N.; Dey, N.; Bera, A.; Mariappan, M.M.; Kasinath, B.S.; Ghosh-Choudhury, G. High glucose forces a positive feedback loop connecting Akt kinase and FoxO1 transcription factor to activate mTORC1 kinase for mesangial cell hypertrophy and matrix protein expression. J. Biol. Chem. 2014, 289, 32703–327016. [Google Scholar] [CrossRef] [PubMed]
- Lüpertz, R.; Chovolou, Y.; Kampkötter, A.; Wätjen, W.; Kahl, R. Catalase overexpression impairs TNF-α induced NF-κB activation and sensitizes MCF-7 cells against TNF-α. J. Cell. Biochem. 2008, 103, 1497–1511. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.J.J.; Miceli, M.V.; Newsome, D.A. Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1271–1279. [Google Scholar]
- He, M.H.; Zhang, Y.; Bao, S. Advanced glycation end products inhibit glucose-6-phosphate dehydrogenase activity and expression in human umbilical vein endothelial cells. Acta Physiol. Sin. 2012, 64, 646–650. [Google Scholar]
- Leopold, J.A.; Zhang, Y.Y.; Scribner, A.W.; Stanton, R.C.; Loscalzo, J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Gosset, P.; Wallaert, B.; Tonnel, A.B.; Fourneau, C. Thiol regulation of the production of TNF-α, IL-6 and IL-8 by human alveolar macrophages. Eur. Respir. J. 1999, 14, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, F.; Mocali, A. Determination of superoxide dismutase activity by purely chemical system based on NADPH oxidation. Methods Enzymol. 1990, 186, 209–221. [Google Scholar] [PubMed]
- Aebi, H. Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 673–677. [Google Scholar]
- Beutler, E. Red cell metabolism. In A Manual of Biochemical Methods; Beutler, E., Ed.; Grune and Stratton: Orlando, FL, USA, 1984; pp. 68–73. [Google Scholar]
- Goldberg, D.M.; Spooner, R.J. Glutathione reductase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1983; Volume 111, pp. 258–265. [Google Scholar]
- Lohr, G.W.; Waller, H.D. Glucose-6-phosphate dehydrogenase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 744–751. [Google Scholar]
- Del Rio, D.; Pellegrini, N.; Colombi, B.; Bianchi, M.; Serafini, M.; Torta, F.; Tegoni, M.; Musci, M.; Brighenti, F. Rapid fluorimetric method to detect total plasma malondialdehyde with mild derivatization conditions. Clin. Chem. 2003, 49, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Chomezynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 1993, 15, 532–537. [Google Scholar]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- NCBI Database. Available online: http://www.ncbi.nlm.nih.gov/nuccore (accessed on 27 April 2015).
- Untergrasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, A.I.; Stanca, L.; Geicu, O.I.; Dinischiotu, A. AGEs-Induced IL-6 Synthesis Precedes RAGE Up-Regulation in HEK 293 Cells: An Alternative Inflammatory Mechanism? Int. J. Mol. Sci. 2015, 16, 20100-20117. https://doi.org/10.3390/ijms160920100
Serban AI, Stanca L, Geicu OI, Dinischiotu A. AGEs-Induced IL-6 Synthesis Precedes RAGE Up-Regulation in HEK 293 Cells: An Alternative Inflammatory Mechanism? International Journal of Molecular Sciences. 2015; 16(9):20100-20117. https://doi.org/10.3390/ijms160920100
Chicago/Turabian StyleSerban, Andreea Iren, Loredana Stanca, Ovidiu Ionut Geicu, and Anca Dinischiotu. 2015. "AGEs-Induced IL-6 Synthesis Precedes RAGE Up-Regulation in HEK 293 Cells: An Alternative Inflammatory Mechanism?" International Journal of Molecular Sciences 16, no. 9: 20100-20117. https://doi.org/10.3390/ijms160920100
APA StyleSerban, A. I., Stanca, L., Geicu, O. I., & Dinischiotu, A. (2015). AGEs-Induced IL-6 Synthesis Precedes RAGE Up-Regulation in HEK 293 Cells: An Alternative Inflammatory Mechanism? International Journal of Molecular Sciences, 16(9), 20100-20117. https://doi.org/10.3390/ijms160920100